Reversible and irreversible processes v Idea of entropy

Reversible and irreversible processes v Idea of entropy v The 2 nd law of thermodynamics v 普通物理學甲上 (202 101 A 1) General Physics(A)(1) 台大物理 吳俊輝

Direction of time v Why time has direction? Why one-way processes are irreversible From energy conservation? v. Hot coffee cup and cold hand get colder hands? v Entropy postulate If an irreversible process occurs in a closed system, the entropy S of the system always increases; it never decreases.
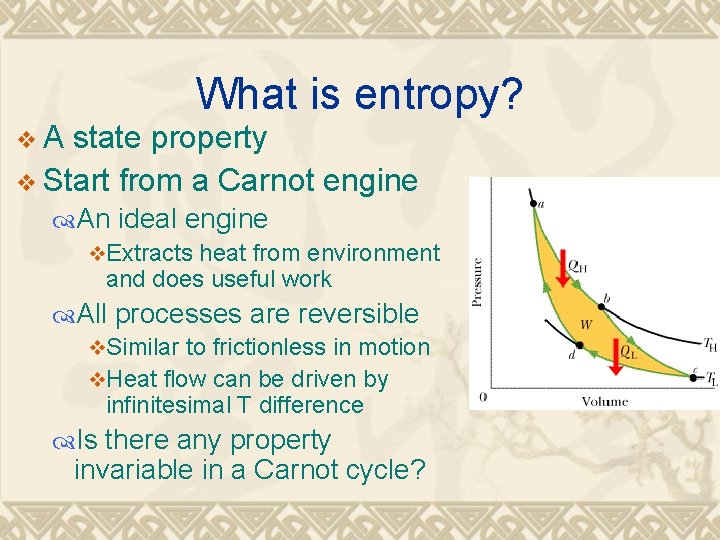
What is entropy? v. A state property v Start from a Carnot engine An ideal engine v. Extracts heat from environment and does useful work All processes are reversible v. Similar to frictionless in motion v. Heat flow can be driven by infinitesimal T difference Is there any property invariable in a Carnot cycle?
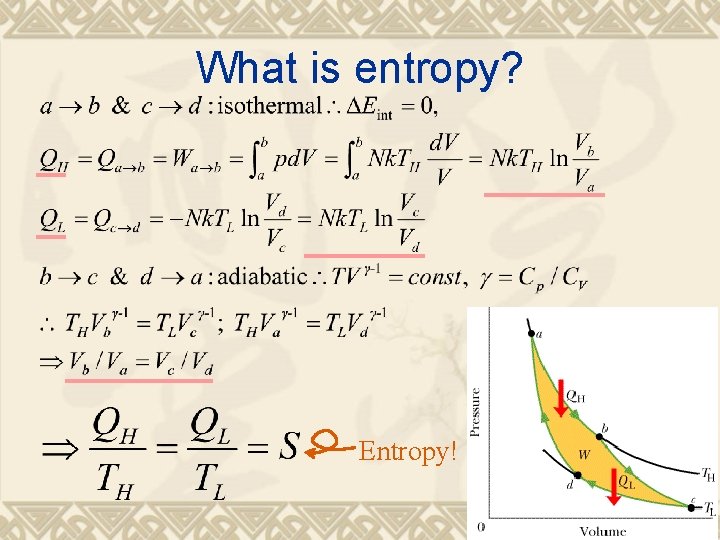
What is entropy? Entropy!
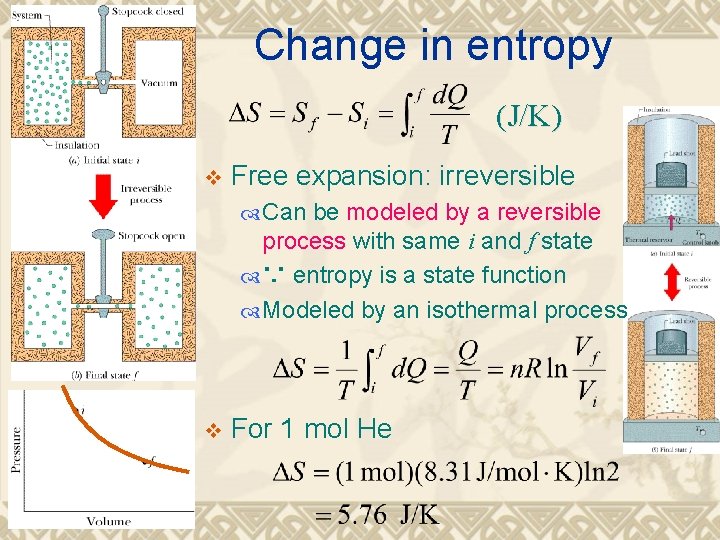
Change in entropy (J/K) v Free expansion: irreversible Can be modeled by a reversible process with same i and f state ∵ entropy is a state function Modeled by an isothermal process v For 1 mol He

If we reverse the process… v Decreasing entropy!! Violate our entropy postulate? We have to consider a closed system
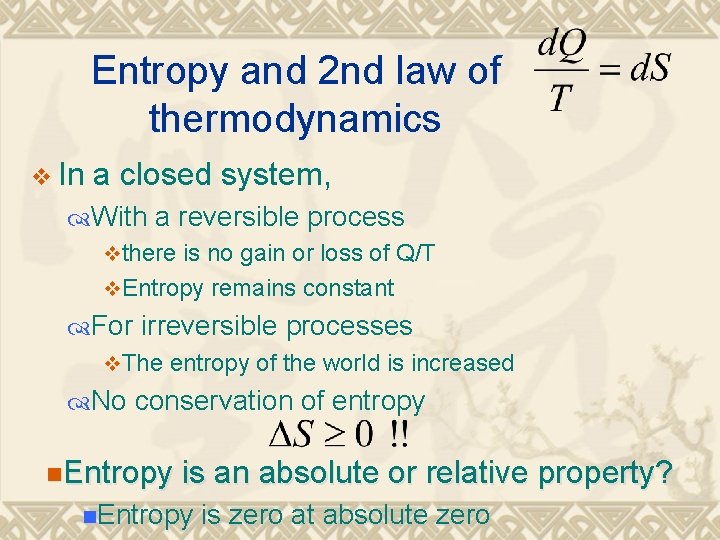
Entropy and 2 nd law of thermodynamics v In a closed system, With a reversible process vthere is no gain or loss of Q/T v. Entropy remains constant For irreversible processes v. The entropy of the world is increased No conservation of entropy n. Entropy is an absolute or relative property? n. Entropy is zero at absolute zero

Change in entropy v What is DS of a popped popcorn? 1. Water in pericarp vaporized at 180 o. C (water mass = 4 mg) 2. Adiabatic expansion of the vapor Corresponding to each audible pop http: //www. buetzer. info/fileadmin/pb/HTML-Files/Popcorn. htm

Example of irreversibility v Work done on an object by friction Q = W Entropy of the world increase by W/T v Hot stone in cold water DS of stone? v–Q/T 1 DS of water? v. Q/T 2 DS of the world? v. Q/T 2–Q/T 1>0
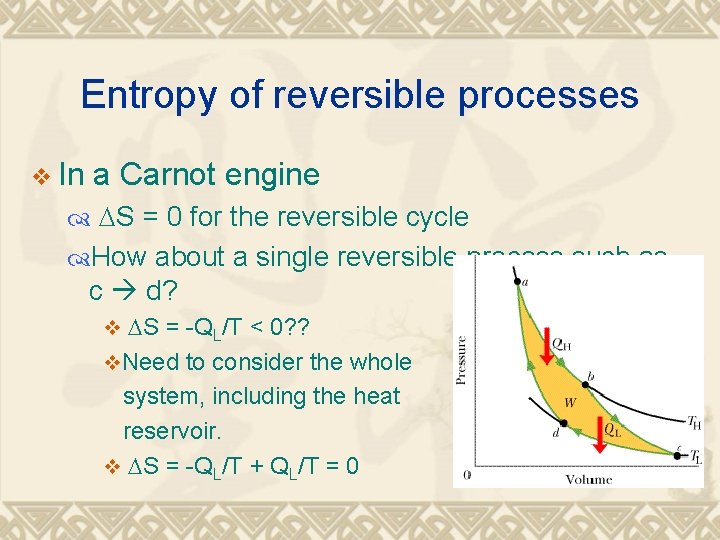
Entropy of reversible processes v In a Carnot engine DS = 0 for the reversible cycle How about a single reversible process such as c d? v DS = -QL/T < 0? ? v. Need to consider the whole system, including the heat reservoir. v DS = -QL/T + QL/T = 0

Entropy and time v Entropy is the arrow of time For irreversible processes, the entropy always increases the forward direction of time To return in time requires an entropy decrease v. Not allowed by thermodynamics

v Ch 19: v Deadline: 1/8, 10: 20 AM

v 1/13 10: 20 -12: 10 教室 v 70號之前在 物理 104 v 1/15 10: 20 -12: 10 教室 v 70號之後在 物理 304
- Slides: 13