RET 300 Congenital Heart Defects The Normal Heart





























- Slides: 94

RET 300 Congenital Heart Defects

The Normal Heart

The Fetal Heart

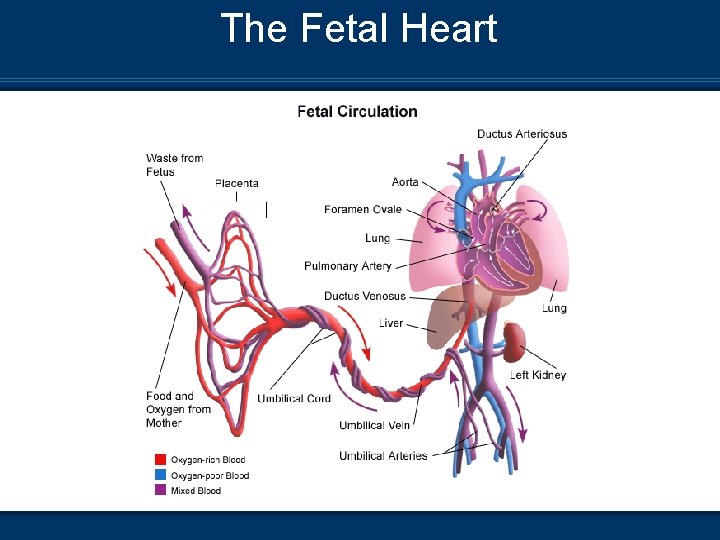
Fetal Circulation Blood travels from the placenta through the umbilical cord via the Umbilical Vein (UV) to the fetus n This blood is effectively the baby’s arterial blood in that it is oxygen- and nutrient-rich and scrubbed of waste metabolites and carbon dioxide n This vessel is used after delivery as a site for venous access and delivery of medications and volume expanders n The UV enters the liver where about ½ of the flow supplies the organ and the other ½ flows through the ductus venosus to the inferior vena cava n

Fetal Circulation n After entering the inferior vena cava, blood mixes in the right atrium with blood from the superior vena cava (deoxygenated blood) n Most of the blood then flows through the foramen ovale into the left atrium n A small amount (~10 -15%) of additional deoxygenated blood from the pulmonary veins enters the left atrium, further decreasing the overall saturation before being pumped out of the left ventricle to provide systemic perfusion

Fetal Circulation n The blood that is not involved in this normally occurring shunt enters the right ventricle, where it is pumped into the pulmonary artery. Of this, only about 10 - 15% actually passes through the lung and the rest is shunted through the ductus arteriosis into the aorta

Transition from Fetal Circulation n At birth, several changes are required to support extra -uterine life • • n Fluid-filled lungs are air-aerated by the removal of fetal lung fluid by the pulmonary circulation and lymphatics, and squeezing during delivery With the aid of surfactant to maintain some FRC, pulmonary gas exchange results in elevated PO 2’s (must be at least 4550 mm. Hg). This causes vasodilation of the pulmonary circulation and constriction of the ductus arteriosis Blood from the right heart now follows the normal adult course through the lungs

Transition from Fetal Circulation n The consequent fall in right atrial pressures causes the pressure gradient across the foramen ovale to reverse • • Functional closure within hours ~20% of adults have some degree of patent foramen ovale that is generally of no consequence n Removal of blood flow through the ductus venosus as the placenta is removed from the equation results in rapid closure of this vessel n The closure of the ductus venosus and loss of the placenta results in an increase in SVR

The Fetal Heart

Transition from Fetal Circulation n As the placenta dramatically ceases to function, p. H and PO 2 fall and PCO 2 rises. The exact mechanism is unknown; however, the central and peripheral chemoreceptors acutely increase in sensitivity and a stimulus to take the first breath is triggered n The increase in PO 2 causes dilation of the pulmonary vascular bed, resulting in a reduction in PVR n SVR is now greater than PVR and right to left shunting is decreased

Fetal Circulation – Shunts n Ductus Venosus • Becomes the Ligamentum Venosum, a fibrous cord in the liver n Foramen Ovale • Becomes a depression in the atrial septum called the Fossa Ovalis n Ductus Arteriosis • • Atrophies into the ligamentum arteriosum, a fibrous cord between the PA and aorta Occurs between 24 hrs - 2 weeks postpartum

Postpartum Circulation n Blood flow through the foramen ovale and the ductus arteriosus is dependent on the balance between PVR and SVR n This balance can be greatly affected by the presence of congential heart defects n These CHDs can be classified as either • • Cyanotic – Right to left shunting Acyanotic – Left to right shunting

Congenital Heart Defects n Acyanotic – CHDs include 1. 2. 3. 4. 5. 6. Patent Ductus Arteriosus (PDA) Atrial Septal Defect (ASD) Ventricular Septal Defect (VSD) Atrioventricular Septal Defect (AVSD) Aortic Stenosis Coarctation of the Aorta

Congenital Heart Defects n Cyanotic – CHDs include 1. 2. 3. 4. 5. 6. 7. Total Anomalous Pulmonary Venous Return (TAPVR) Tetralogy of Fallot (TEF) Truncus Arteriosus Transposition of the great vessels Tricuspid Atresia Hypoplastic Left Heart Syndrome (HLHS) Pulmonary Stenosis

Acyanotic Heart Defects

Patent Ductus Arteriosus n Most common in premies n L-R shunt

Patent Ductus Arteriosus

Patent Ductus Arteriosus Etiology n Postpartum, the Ductus Arteriosus (DA) closes as a result of hormonal, chemical, and blood gas changes n This shifts the blood flow through the DA from right to left, to left to right (Aorta – Pulmonary artery) n Function of the constrictor response of oxygen to the DA and the dilator effect of prostaglandin are functions of gestational age n More premature, the greater the delay in closure of the DA

Patent Ductus Arteriosus Pathophysiology n Failure of the constrictor responses results in an n n increase in pulmonary blood flow through the PDA Presence of PDA results in pulmonary overflow, resulting in pulmonary congestion Pulmonary hypertension and congestive heart failure may follow Impairs lung function and compliance If untreated, it leads to reversal of the shunt back to right to left from an increase in PVR

Patent Ductus Arteriosus Clinical Manifestations n Preductal and postductal assessment of oxygenation n n either invasively or non-invasively Should result in a difference of greater than 15 mm. Hg or 5 - 10% in saturation CXR may result in increased vascular markings if congestive heart failure develops Definitive diagnosis is done by echocardiology determining the presence of the PDA Normal ABGs and pulse oximetry can be expected post-repair

Patent Ductus Arteriosus Management/Treatment n Infants are kept euvolemic with high end of normal n n hemoglobin levels Treatment includes a trial of indomethacin given in the first 24 hrs postpartum – reduces the risk of a PDA developing Use of PEEP may assist in reducing the shunt Surgical repair of the PDA will reduce the duration of ventilatory support PDA ligation

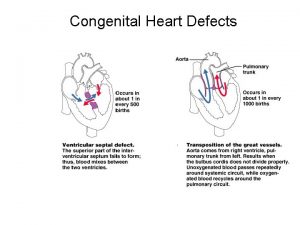
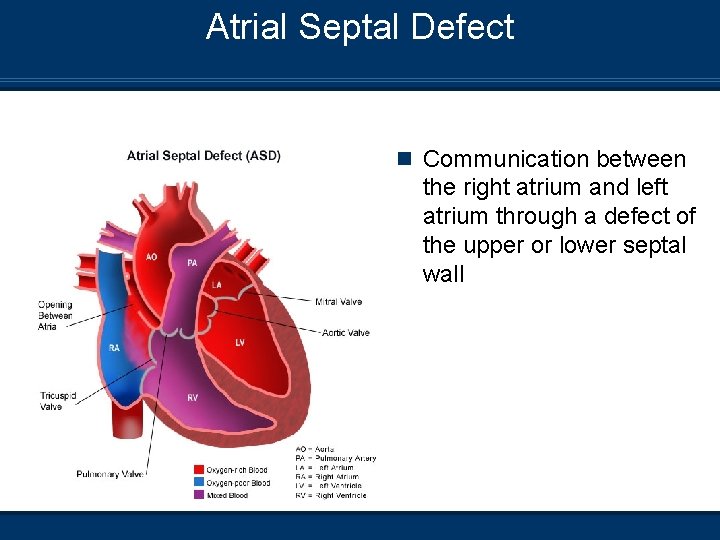
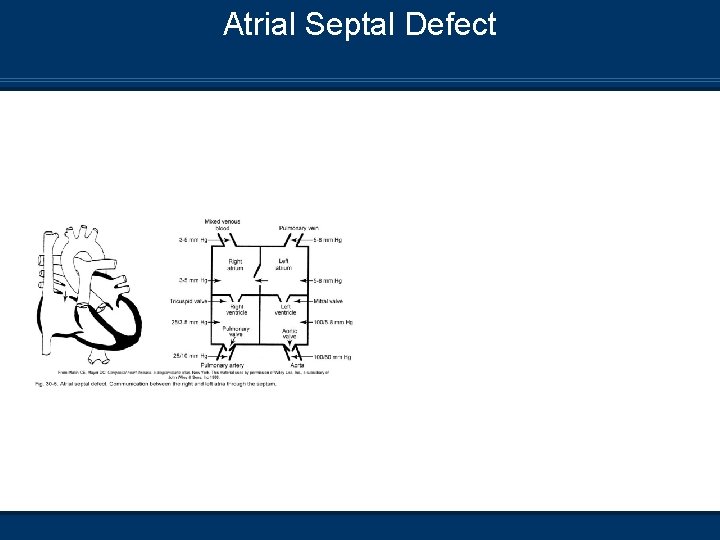
Atrial Septal Defect n Communication between the right atrium and left atrium through a defect of the upper or lower septal wall

Atrial Septal Defect

Atrial Septal Defect (ASD) Etiology n The communication develops from 1. 2. 3. Incompetent foramen ovale Developmental defect in the septum Failure to develop the endocardial cushion n Three types of ASD may present 1. Primum (lower) 2. Secundum (Central) 3. Sinus Venosus (Upper)

Atrial Septal Defect Pathophysiology n Left to right shunting of blood occurs n Rarely produces pulmonary congestion n May produce right ventricular hypertrophy from pulmonary overflow

Atrial Septal Defect – Clinical Manifestations n CXR are usually normal unless there is pulmonary congestion – An increase in vascular markings n Most children with an ASD will not become symptomatic until school age n This may manifest as SOB on exertion n Chest palpitations n Frequent pulmonary infections n Dyspnea n Normal ABGs and pulse oximetry can be expected post-repair n

Atrial Septal Defect Management/Treatment n Diagnosis is usually made by Echocardiogram n Cardiac catheterization n n Repair is dependent on the condition of the child and the degree of pulmonary involvement and cardiomegaly n Open chest surgical repair will require cardiopulmonary bypass n ASD repair by cardiac catheterization

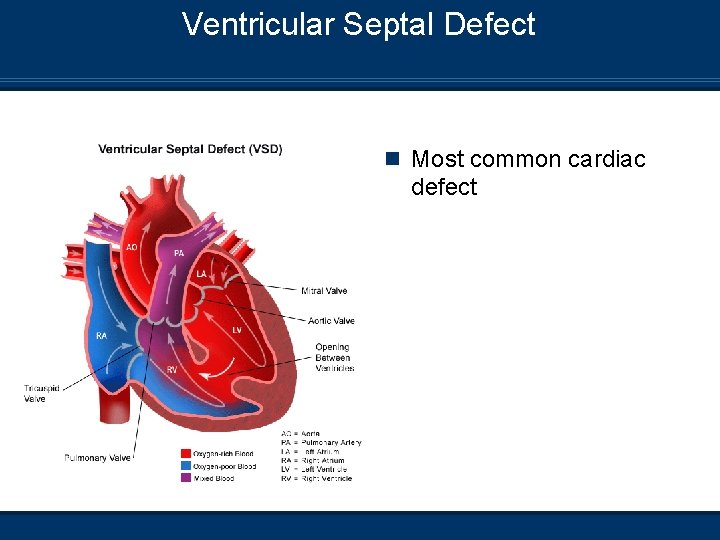
Ventricular Septal Defect n Most common cardiac defect

Ventricular Septal Defect

Ventricular Septal Defect (VSD) Etiology n A communication between the right and left ventricles n Size may range from a pinhole to absence of the septal wall n The majority of small VSDs close without intervention between the ages of 1 and 2

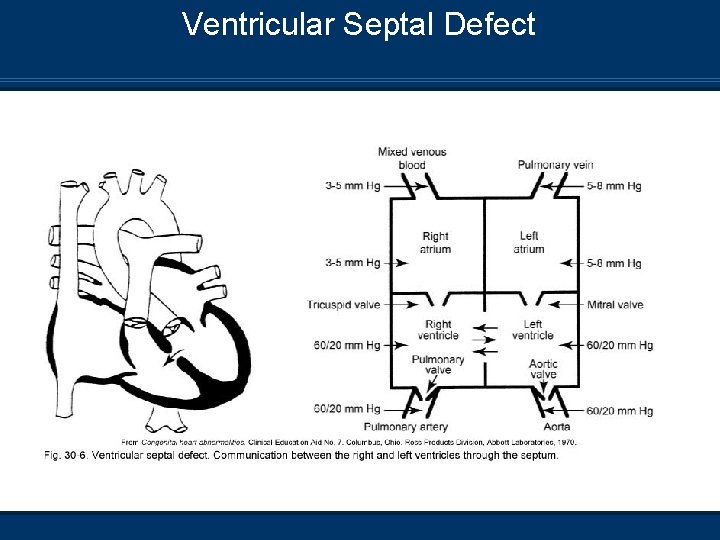
Ventricular Septal Defect Pathophysiology n VSD results in left to right shunting across the n n opening following the pressure gradient Recalling that PVR is less than SVR Shunting of blood primarily occurs during the systolic period Increased pulmonary blood flow may lead to thickening and fibrosis of the pulmonary arterioles (Permanent damage) Leads to pulmonary hypertension and congestive heart failure

Ventricular Septal Defect Clinical Manifestations n CXR Small VSD may not show anything on CXR n Larger VSD may have increased cardiac shadow n Increased pulmonary vascular markings if congestive heart failure develops n n Pa. O 2 and saturation will be on the low end of normal n Children may have poor growth development n Pulmonary hypertension

Ventricular Septal Defect Management/Treatment n Medical management may include digoxin and furosemide n Dacron banding of the pulmonary artery root to allow child to grow for permanent repair n Surgical repair depends on the degree of the VSD Suturing closed for small VSD n Patch sewn into place for the larger VSD n Surgical Repair of ASD and VSD n Normal ABGs and pulse oximetry can be expected post-repair n

Atrioventricular Septal Defect

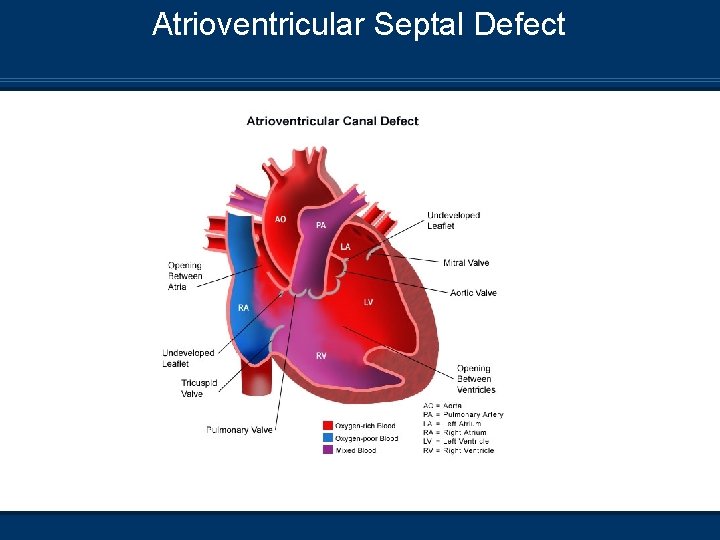
Atrioventricular Septal Defect

Atrioventricular Septal Defect Pathophysiology n Incomplete development of both the atrial septal walls n n n and ventricular septal walls No tricuspid or mitral valves formed Single large valve forms across the defect L-R shunt via ASD and VSD Most common in Down’s Syndrome If severe, seen at 1 - 2 wks Mixing of atrial and ventricular blood

Atrioventricular Septal Defect Clinical Manifestations n CXR Cardiomegaly and increased pulmonary vascular markings n Congestive heart failure n n Oxygenation n Saturations usually run between 75 - 90%

Atrioventricular Septal Defect Management/Treatment n Oxygenation n Supplemental oxygen should be used cautiously to prevent pulmonary vasodilation n Pulmonary artery banding to increase PVR – Temporary fix until infant has increased in size for surgical correction n Surgical correction of ASD and VSD with formation of tricuspid and mitral valves n Normal ABG values can be expected if complete surgical repair is done

Atrioventricular Septal Defect Management/Treatment n Care is taken to not disrupt the AV conduction system, but pacing wires are usually in place for the postoperative period to prevent arrhythmias n Pulmonary HTN may develop postoperatively, leading to an increased risk in mortality n If PA banding is performed, there is increased risk for mixing of deoxygenated and oxygenated blood n Pa. O 2 = 40 - 60 mm. Hg and Sa. O 2 = 75 - 85%

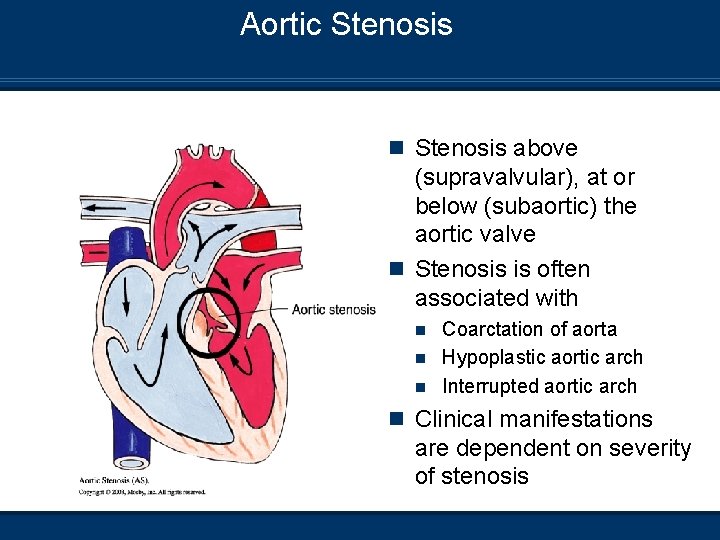
Aortic Stenosis n Stenosis above (supravalvular), at or below (subaortic) the aortic valve n Stenosis is often associated with Coarctation of aorta n Hypoplastic aortic arch n Interrupted aortic arch n n Clinical manifestations are dependent on severity of stenosis

Aortic Stenosis

Aortic Stenosis Pathophysiology n Myocardial hypertrophy and ventricular overdistention n n related to restriction in flow Increased pressure in LV Decreased CO LHF backup of pressure can cause RHF Systemic blood flow – Ductal-dependent

Aortic Stenosis Clinical Manifestations n CXR Congestive heart failure with enlarged cardiac silhouette n Increased pulmonary markings – pulmonary venous congestion n n Infants often are acidotic and hypoxic n Decreased cardiac output n Decreased systemic BP n Chest pain – Tachycardia n Syncope

Aortic Stenois Management/Treatment n Prostaglandin E 1 n Supplement oxygen to support oxygenation n Cardiac catheterization – Valvotomy n Surgical intervention Aortic valvoplasty n Replacement with artificial valve n Pulmonary valve autograph (Ross Procedure) n Widening of atenotic area n n Ventilation to support WOB and acidosis

Aortic Stenosis Management/Treatment n Normal ABGs and pulse oximetry post-correction n Limiting systemic BP postoperatively n Ross Procedure – Aortic valve replacement

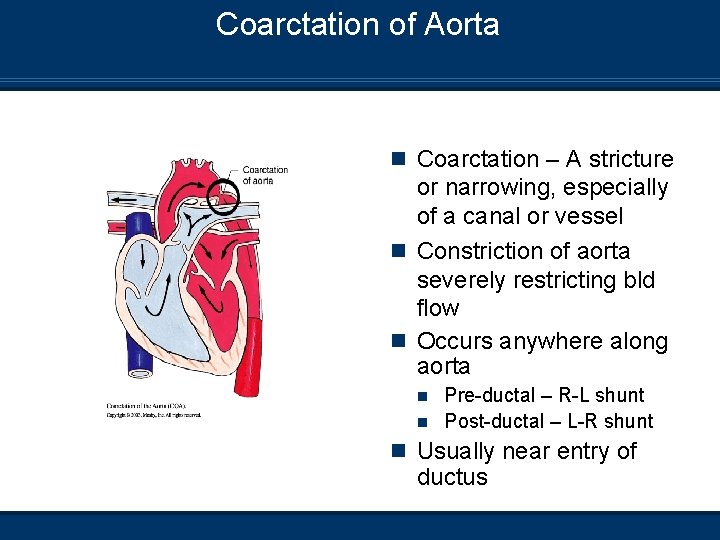
Coarctation of Aorta n Coarctation – A stricture or narrowing, especially of a canal or vessel n Constriction of aorta severely restricting bld flow n Occurs anywhere along aorta Pre-ductal – R-L shunt n Post-ductal – L-R shunt n n Usually near entry of ductus

Coarctation of Aorta

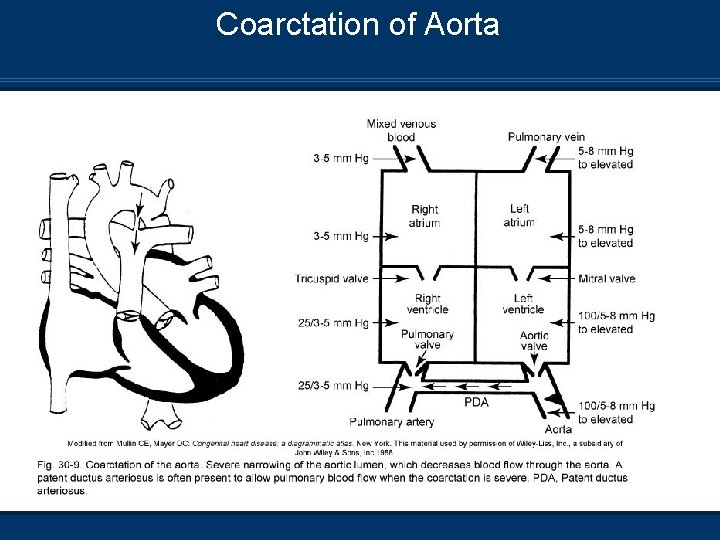
Coarctation of Aorta Pathophysiology n Severe narrowing of the aorta – Decreased blood flow n n distal to the stricture Increased LV work and filling pressures Increased LV outflow resistance LV hypertrophy may occur if DA closes before the coarctation is identified RV hypertrophy in infancy

Coarctation of Aorta Pathophysiology n Preductal narrowing Associated with VSD and aortic stenosis n Descending aorta perfused by RV via ductus arteriosis (right to left shunt) n Closure of DA results in decrease in systemic perfusion (Shock – like presentation) and renal shutdown n Postductal narrowing n May develop collateral circulation n

Coarctation of Aorta Clinical Manifestations n CXR Enlarged heart shadow n Pulmonary vascular congestion Dyspnea Tachycardia Hypertension – Upper extremities Poor BP in lower extremities n n n

Coarctation of Aorta Management/Treatment n Inotropic support for decreased BP and cardiac output n Ventilatory support for the patient presenting with shock n Prostaglandin E 1 n Diuretic therapy – Congestive heart failure

Coarctation of Aorta Management/Treatment n Surgical correction once patient is able to tolerate Excision of the coarctated segment aorta n Tubular graft bypass of the coarctated n Patch aortoplasty from the subclavian artery n n Normal ABGs and oximetry can be expected n May develop postoperative rebound hypertension n Coarctation of aorta repair

Cyanotic Heart Defects

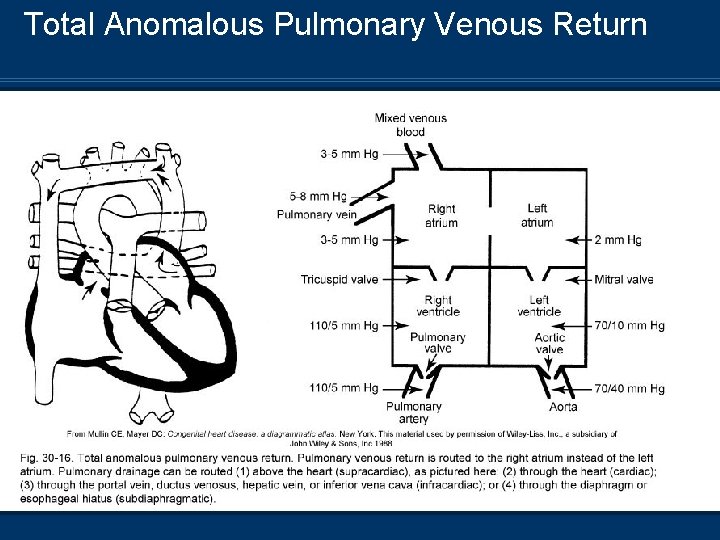
Total Anomalous Pulmonary Venous Return n Pulmonary veins no connection with LA; they drain through multiple alternate routes 1. Supracardiac 2. Cardiac 3. Infracardiac 4. Mixed n Requires PFO, ASD, or VSD for survival

Total Anomalous Pulmonary Venous Return

Total Anomalous Pulmonary Venous Return

Total Anomalous Pulmonary Venous Return Pathophysiology n Mixing of pulmonary and systemic blood – Venous n n admixture Increased blood volume and pressures in the RA Pulmonary overflow and increased right to left shunt from PDA Systemic oxygenation is dependent on right to left shunt through ASD or PFO Infracardiac defect may result in pulmonary vein obstruction

Total Anomalous Pulmonary Venous Return Clinical Manifestations n CXR Relatively normal heart shadow n May have increased pulmonary vascular markings with severe obstruction n n ABGs Pa. O 2 = 40 mm. Hg n Pa. CO 2 = 40 mm. Hg n Sp. O 2 = 75% n n Cyanosis – Degree is dependent on severity of obstruction n Poor exertional tolerance – Dyspnea and tachypnea

Total Anomalous Pulmonary Venous Return Management/Treatment n All TAPVR must be surgically repaired soon after diagnosis (May include neonatal period) n Balloon atrial septoplasty – Enlarge ASD n Oxygen therapy – May have refractory hypoxia n Postoperative ventilation – Normal blood gases n Pulmonary edema may prolong ventilation n Postoperative pulmonary hypertension n Total anomalous pulmonary venous return

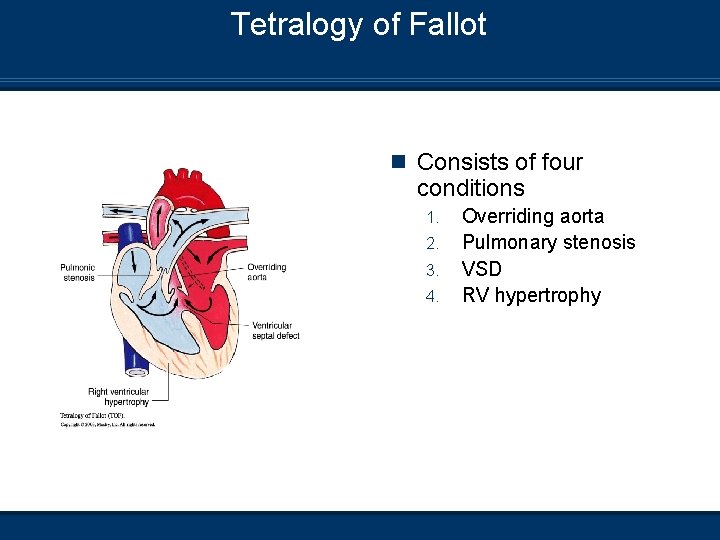
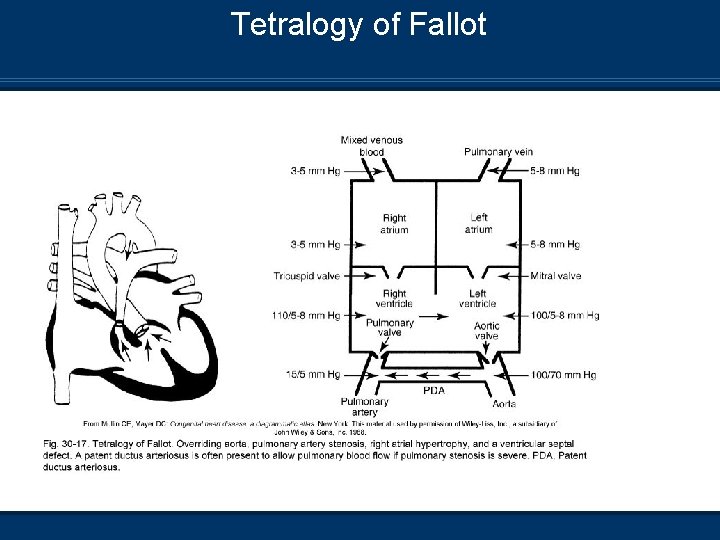
Tetralogy of Fallot n Consists of four conditions 1. 2. 3. 4. Overriding aorta Pulmonary stenosis VSD RV hypertrophy

Tetralogy of Fallot

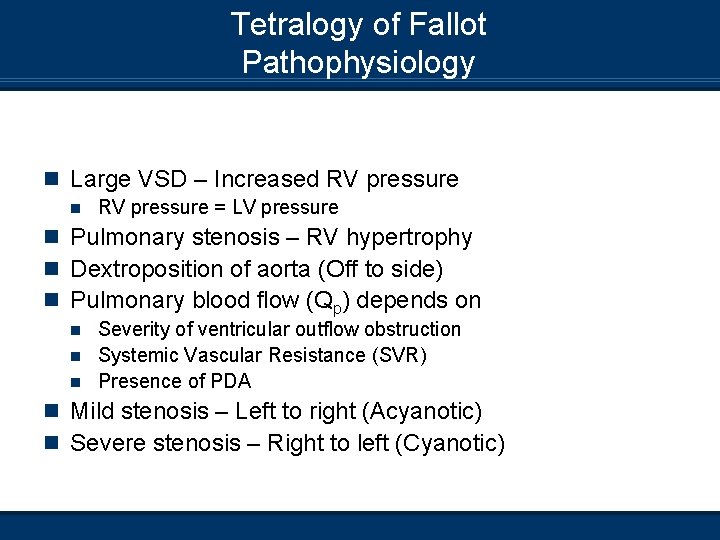
Tetralogy of Fallot Pathophysiology n Large VSD – Increased RV pressure n RV pressure = LV pressure n Pulmonary stenosis – RV hypertrophy n Dextroposition of aorta (Off to side) n Pulmonary blood flow (Qp) depends on Severity of ventricular outflow obstruction n Systemic Vascular Resistance (SVR) n Presence of PDA n n Mild stenosis – Left to right (Acyanotic) n Severe stenosis – Right to left (Cyanotic)

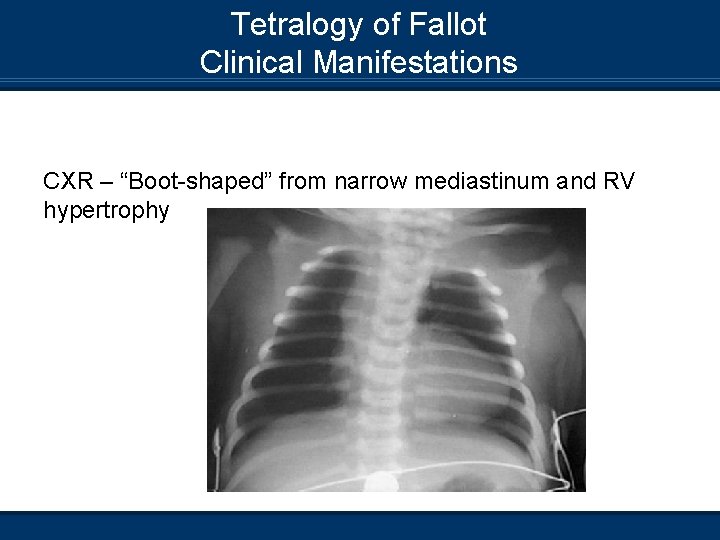
Tetralogy of Fallot Clinical Manifestations CXR – “Boot-shaped” from narrow mediastinum and RV hypertrophy

Tetralogy of Fallot Clinical Manifestations n “TET” spells (2 - 4 months of age) n n n Hyperpnea or exaggerated breathing Irritability and prolonged crying Increasing cyanosis – Increased PVR – Increases R to L shunting = increased hypoxia Decreased intensity of the heart murmur Fainting n Treatment of “TET” spells n n n Hold in knee-to-chest position Morphine sulphate – Decrease respiratory drive Administer oxygen Sodium Bicarbonate – Acidosis Vasoconstrictors – Increase SVR Minimize interventions that precipitate “TET” spell

Tetrology of Fallot Management/Treatment n Prostaglandin E 1 – Maintain PDA n Oxygen therapy n Surgical correction done under cardiopulmonary bypass Widening of RV outflow tract n Reconstruction of stenotic pulmonary artery n Closure of VSD n Blalock-Taussig shunt to improve systemic to pulmonary circulation (Severe stenosis) n n Tetralogy of fallot repair

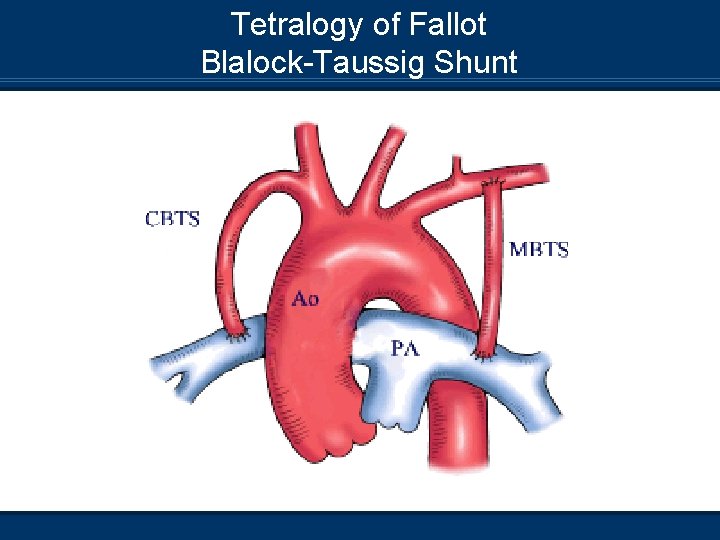
Tetralogy of Fallot Blalock-Taussig Shunt

Tetralogy of Fallot Management/Treatment n Ventilatory support – Postoperative period n Normal ABGs can be expected – Complete repair n Atrial and ventricular pacing wires – Atrial junctional tachycardia

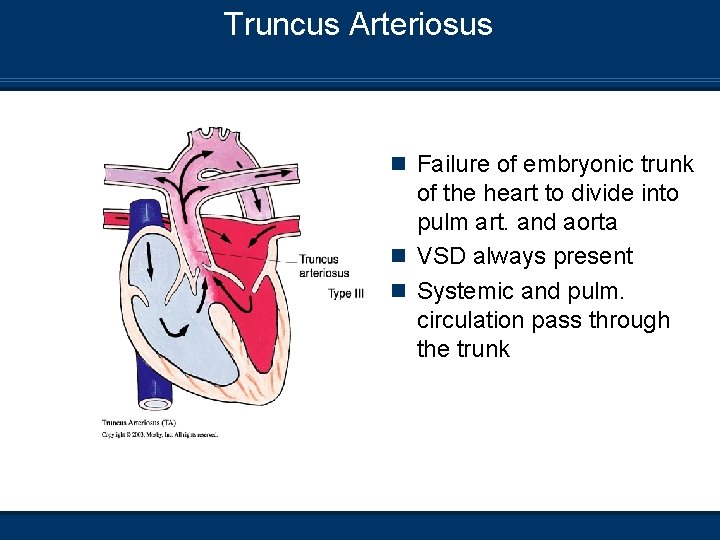
Truncus Arteriosus n Failure of embryonic trunk of the heart to divide into pulm art. and aorta n VSD always present n Systemic and pulm. circulation pass through the trunk

Truncus Arteriosus

Truncus Arteriosus Pathophysiology n Single large artery (truncal vessel) comes from single large ventricle n Large VSD (RV and LV act as one common ventricle) n Truncal valve similar to aortic valve n Systemic circulation and oxygenation is dependent on balance between PVR and SVR If PVR decreases = increase in Qp and Qs decreases n If SVR decreases = increase in Qs and Qp decreases n

Truncus Arteriosus Clinical Manifestations n CXR Enlarged heart shadow – Dilation of LA and LV n Congestive heart failure n n ABGs Rule of 40’s applies n Acidosis n Hypoxemia – Dependent on balance between SVR and PVR n n Tachypnea n Poor feeding and lethargy

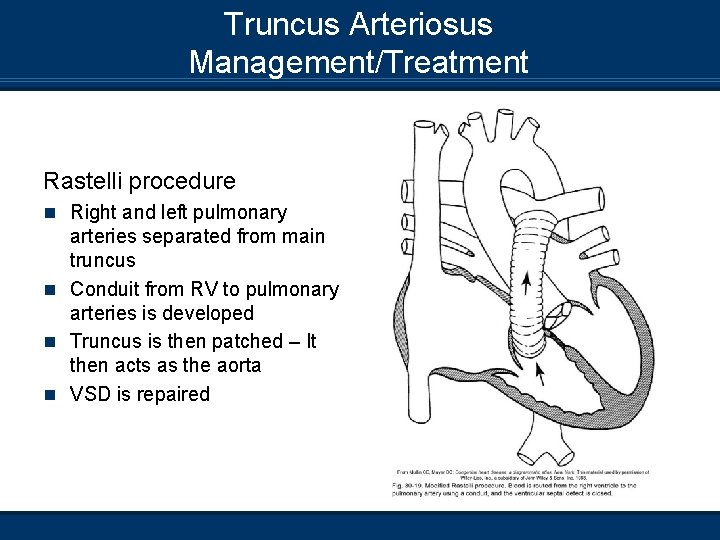
Truncus Arteriosus Management/Treatment n Oxygen therapy – Careful administration to prevent n n n imbalances in PVR Inotropic support – Systemic circulation Diuretics – Maintain an euvolemic state Pulmonary artery banding – Palliative procedure to allow infant to stabilize Surgical correction under cardiopulmonary bypass using a Rastelli procedure Normal ABGs can be expected post-correction Truncus arteriosus repair

Truncus Arteriosus Management/Treatment Rastelli procedure n Right and left pulmonary arteries separated from main truncus n Conduit from RV to pulmonary arteries is developed n Truncus is then patched – It then acts as the aorta n VSD is repaired

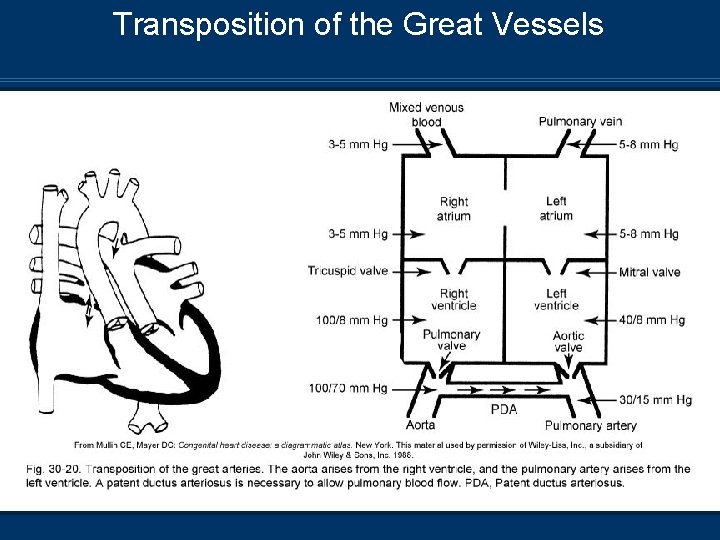
Transposition of the Great Vessels n Two parallel circulations n n Aorta rises from RV Pulmonary artery rises from LV

Transposition of the Great Vessels

Transposition of the Great Vessels Pathophysiology n Right circulation – Deoxygenated blood circulates through RA, RV, aorta, systemic circulation, and back to RA n Left circulation – Oxygenated blood from pulmonary veins has continuous circuit through LA, LV pulmonary circulation, and back to LA n Must have connection between R+L heart ASD n PFO n PDA n n Will result in death if not corrected

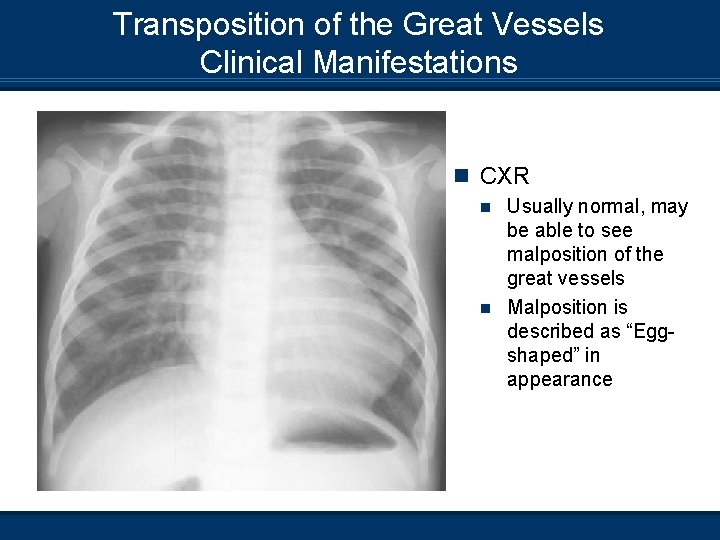
Transposition of the Great Vessels Clinical Manifestations n CXR Usually normal, may be able to see malposition of the great vessels n Malposition is described as “Eggshaped” in appearance n

Transposition of the Great Vessels Clinical Manifestations n ABGs Shunting from aorta to PA, and LA to RA provides adequate mixing of blood n Closure of ductus arteriosus results in severe cyanosis and hypoxemia n Infant will develop severe acidosis and hypercapnia n n Infant will present with Tachypnea n Retractions, grunting, nasal flaring, etc. n

Transposition of the Great Vessels Management/Treatment n Prostaglandin E 1 – Maintain PDA upon recognition of n n abnormality Intubation and ventilation to manage blood gas abnormalities Oxygen therapy – Balance between PVR and SVR Atrial septoplasty of ASD – Increase shunt from RA to LA PA banding to increase LV conditioning to support systemic circulation post-surgical correction

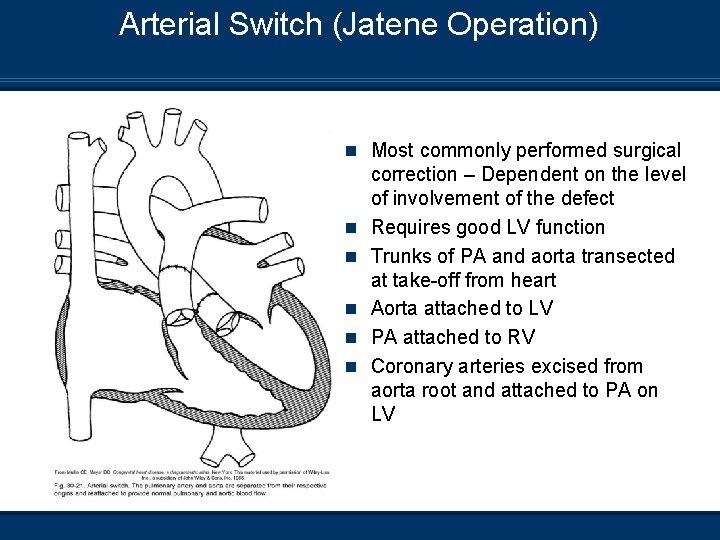
Arterial Switch (Jatene Operation) n Most commonly performed surgical n n n correction – Dependent on the level of involvement of the defect Requires good LV function Trunks of PA and aorta transected at take-off from heart Aorta attached to LV PA attached to RV Coronary arteries excised from aorta root and attached to PA on LV

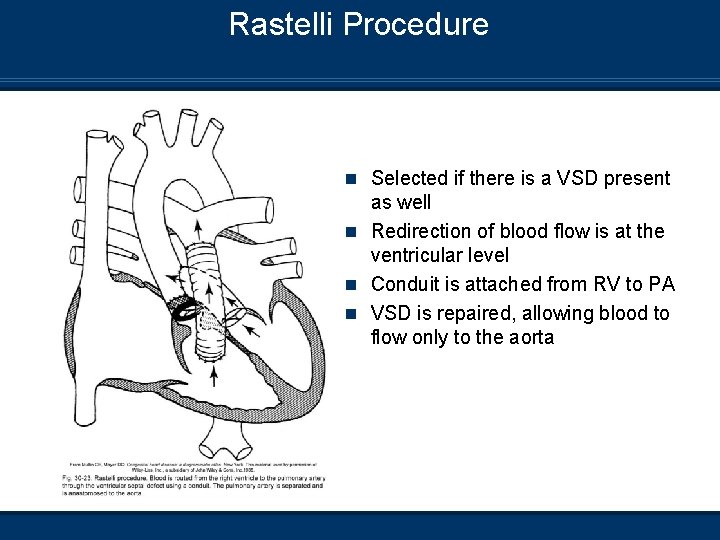
Rastelli Procedure n Selected if there is a VSD present as well n Redirection of blood flow is at the ventricular level n Conduit is attached from RV to PA n VSD is repaired, allowing blood to flow only to the aorta

Transposition of the Great Vessels Management/Treatment n Post-surgical correction; normal blood gases can be n n expected Infants will require inotropic support to balance circulations pre- and post-correction Postoperative ventilation can be expected Arterial switch – Philadelphia Children’s Hospital Arterial switch – Miami Children’s Hospital

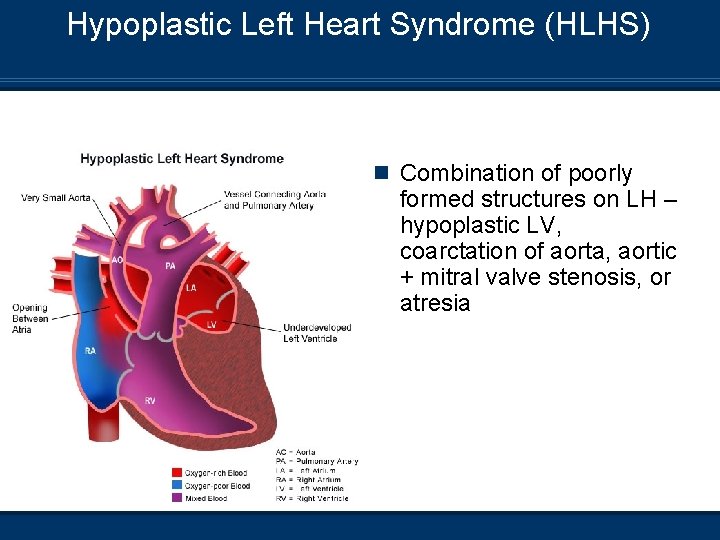
Hypoplastic Left Heart Syndrome (HLHS) n Combination of poorly formed structures on LH – hypoplastic LV, coarctation of aorta, aortic + mitral valve stenosis, or atresia

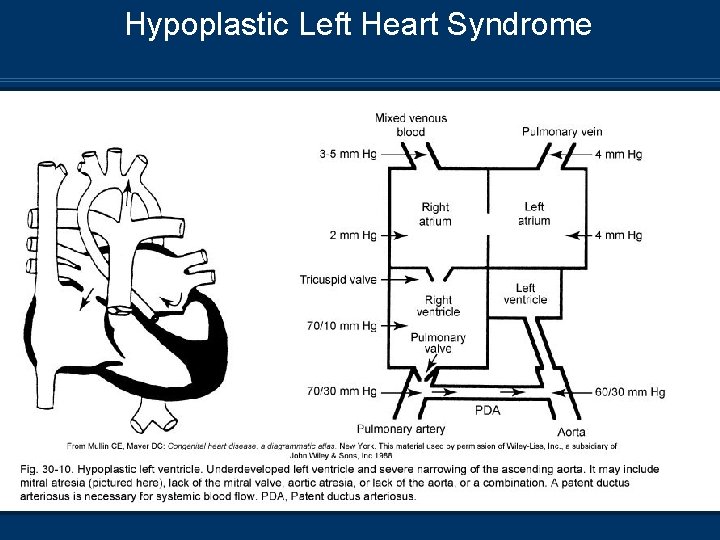
Hypoplastic Left Heart Syndrome

Hypoplastic Left Heart Syndrome Pathophysiology n Shunting occurs at two sites PFO or ASD – L-R shunt through ASD n PDA – R-L shunt through PDA = Systemic blood flow (Systemic blood flow provided by RV) n n Blood flow through LH obstructed = decreased CO n Postnatal closure of DA results in decreased CO Infant will present with circulatory shock n Metabolic acidosis n n Death usually occurs in the first month if not surgically corrected

Hypoplastic Left Heart Syndrome Clinical Manifestations n CXR Will show increased pulmonary vascular markings n Globular shape n n ABGs Hypoxemia n Metabolic acidosis n n Patients may present with cyanosis, tachypnea, grunting, retractions

Hypoplastic Left Heart Syndrome Management/Treatment n Preoperative treatment Prostaglandin E 1 – Maintain PDA n Maintain high PVR relative to SVR – Hypercarbia and acidosis (Permissive hypercapnia and p. H ~ 7. 20) n Maintain Sp. O 2 ~ 70 - 80% n n Mechanical ventilation to manage PVR Addition of mechanical deadspace to ventilator circuit 2. Reduction in minute ventilation 3. Adding CO 2 to the patient circuit 1.

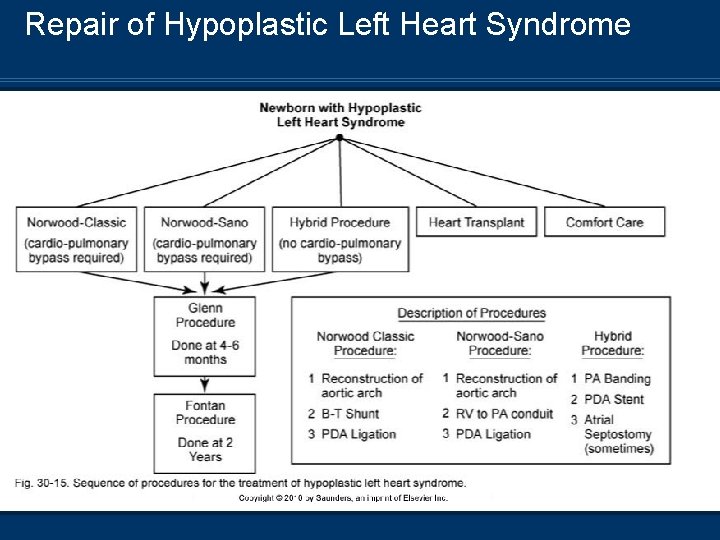
Repair of Hypoplastic Left Heart Syndrome

Stage 1 Repair – Norwood Procedure n Reconstruction of n n the aorta Blalok-Taussig shunt or RV-PA conduit PA banding Atrial septoplasty PDA ligation

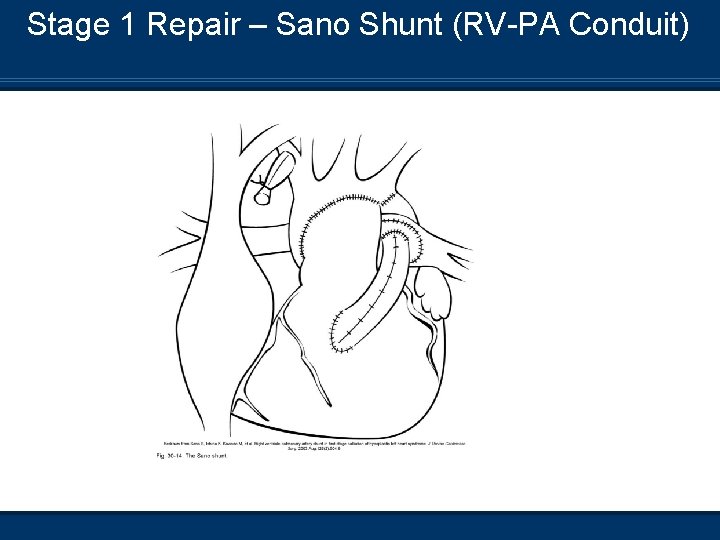
Stage 1 Repair – Sano Shunt (RV-PA Conduit)

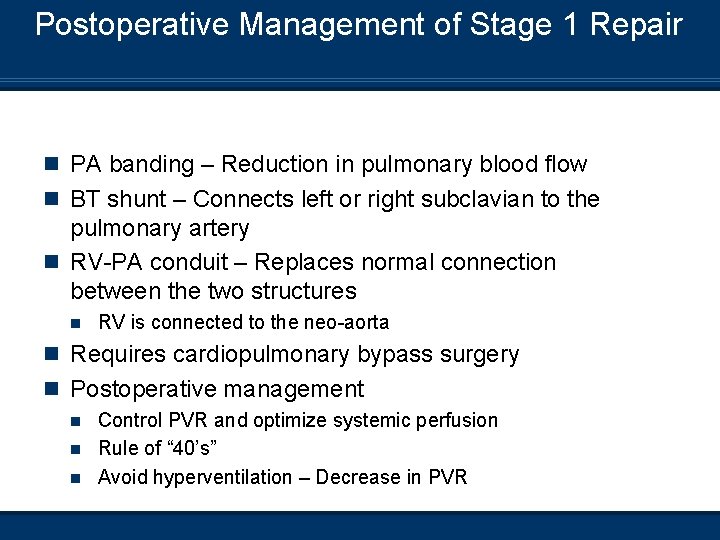
Postoperative Management of Stage 1 Repair n PA banding – Reduction in pulmonary blood flow n BT shunt – Connects left or right subclavian to the pulmonary artery n RV-PA conduit – Replaces normal connection between the two structures n RV is connected to the neo-aorta n Requires cardiopulmonary bypass surgery n Postoperative management Control PVR and optimize systemic perfusion n Rule of “ 40’s” n Avoid hyperventilation – Decrease in PVR n

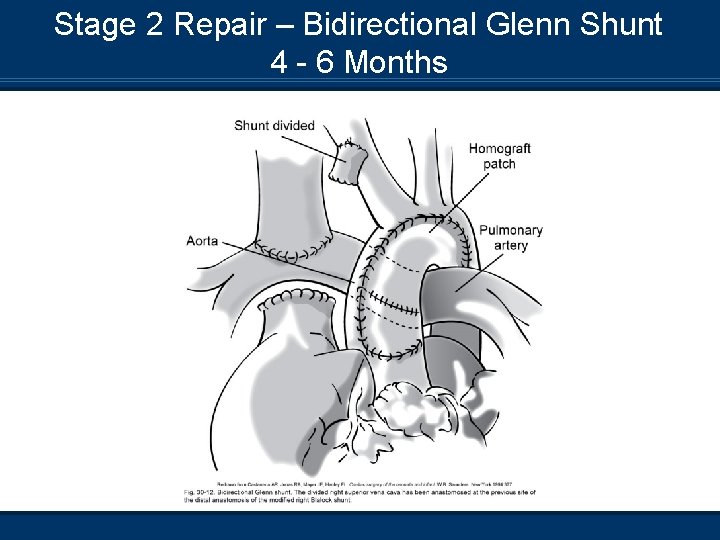
Stage 2 Repair – Bidirectional Glenn Shunt 4 - 6 Months

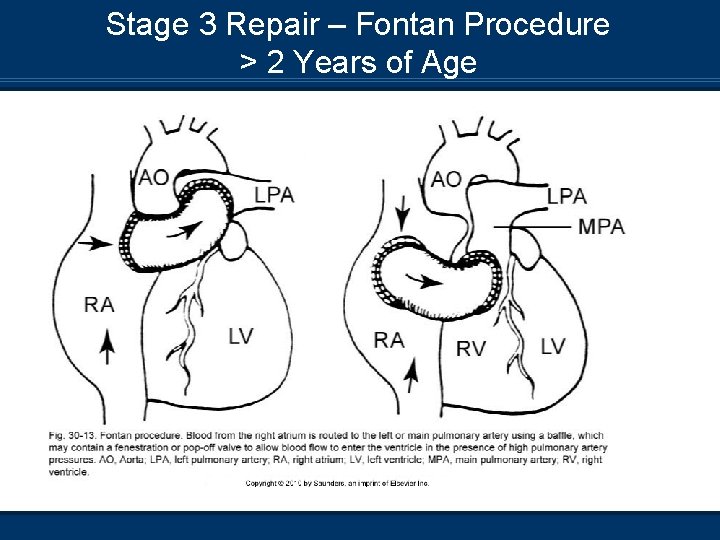
Stage 3 Repair – Fontan Procedure > 2 Years of Age

Postoperative Mangement of Stage 2 and 3 Repairs n Low rate and high tidal volume ventilation n Increase Te = decreased MAP n Early Extubation – Negative intrathoracic pressures n HLHS – Stage 1 repair
 Difference between cyanotic and acyanotic heart disease
Difference between cyanotic and acyanotic heart disease Tetralogy of fallot murmur
Tetralogy of fallot murmur Squatting position in tetralogy of fallot
Squatting position in tetralogy of fallot Canadian congenital heart alliance
Canadian congenital heart alliance Farah garmany
Farah garmany Congenital heart
Congenital heart Congenital heart defect
Congenital heart defect Praiprism
Praiprism Keratocysta
Keratocysta God ret
God ret Xrl in 8051
Xrl in 8051 Ret tapu
Ret tapu Krönika exempel åk 9
Krönika exempel åk 9 Trùng kiết lị
Trùng kiết lị Dalr ret
Dalr ret 300+300+200+200
300+300+200+200 Is 33 prime or composite?
Is 33 prime or composite? 100 200 300
100 200 300 200 300 300
200 300 300 Scientific notation
Scientific notation 300+300+400
300+300+400 300+300+400
300+300+400 300+300+400
300+300+400 300+300+400
300+300+400 400 + 300 + 300
400 + 300 + 300 300 300 400
300 300 400 What the font
What the font 100 + 200 300
100 + 200 300 Ttn vs rds cxr
Ttn vs rds cxr Congenital pneumonia
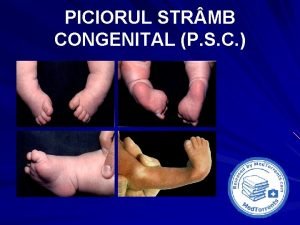
Congenital pneumonia Picior stramb congenital talus valgus
Picior stramb congenital talus valgus Congenital glaucoma classification
Congenital glaucoma classification Congenital rubella syndrome
Congenital rubella syndrome Congenital malformations
Congenital malformations Congenital voice disorders
Congenital voice disorders Hypothyroidism
Hypothyroidism Congenital fibrosis of the extraocular muscles
Congenital fibrosis of the extraocular muscles Pyriform aperture stenosis
Pyriform aperture stenosis Hypobranchial eminence
Hypobranchial eminence Non classical adrenal hyperplasia
Non classical adrenal hyperplasia Congenital rubella syndrome triad
Congenital rubella syndrome triad Endocardial cushion defect
Endocardial cushion defect Congenital rubella syndrome triad
Congenital rubella syndrome triad Congenital adrenal hyperplasia characteristics
Congenital adrenal hyperplasia characteristics Congenital hydronephrosis
Congenital hydronephrosis Congenital hydronephrosis
Congenital hydronephrosis Defect
Defect Congenital adrenal hyperplasia characteristics
Congenital adrenal hyperplasia characteristics Toxoplasmosis
Toxoplasmosis Kode icd 10 clubfoot
Kode icd 10 clubfoot Congenital toxoplasmosis
Congenital toxoplasmosis Congenital anomaly
Congenital anomaly Congenital glaucoma triad
Congenital glaucoma triad Vulvodynia
Vulvodynia Congenital adrenal hyperplasia genitalia
Congenital adrenal hyperplasia genitalia Congenital diaphragmatic hernia
Congenital diaphragmatic hernia Mononucleosis
Mononucleosis Micrognathia definition
Micrognathia definition Spina bifida
Spina bifida Greater and lesser pelvis
Greater and lesser pelvis Congenital adrenal hyperplasia
Congenital adrenal hyperplasia Thyroid storm mnemonic
Thyroid storm mnemonic Barlow sign
Barlow sign Congenital dislocation of hips
Congenital dislocation of hips Congenital
Congenital Congenital amusia
Congenital amusia Hutchinson triad
Hutchinson triad Anal advancement flap
Anal advancement flap Disease penis
Disease penis Congenital
Congenital Congenital glaucoma
Congenital glaucoma Hymen
Hymen Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worm breton là gì
Tư thế worm breton là gì Chúa yêu trần thế
Chúa yêu trần thế Kể tên các môn thể thao
Kể tên các môn thể thao Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ