Responsive Neurostimulation RNS for the treatment of epilepsy





- Slides: 16

Responsive Neurostimulation (RNS) for the treatment of epilepsy Daniel Friedman, MD Assistant Professor NYU Comprehensive Epilepsy Center April 27, 2014

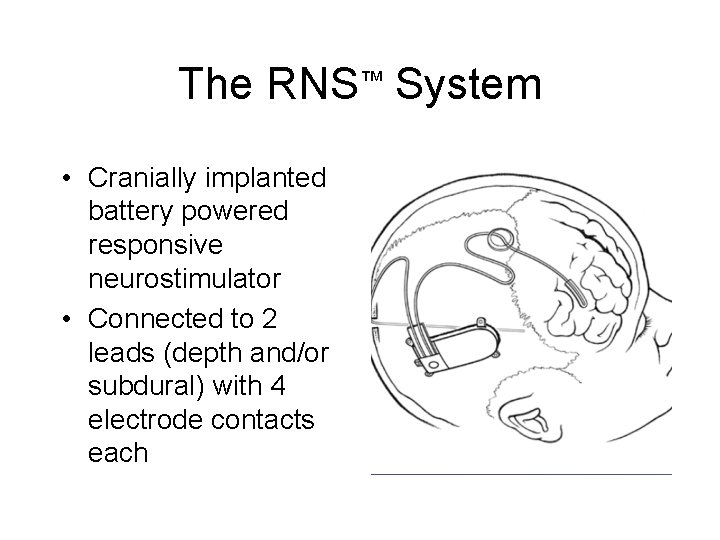
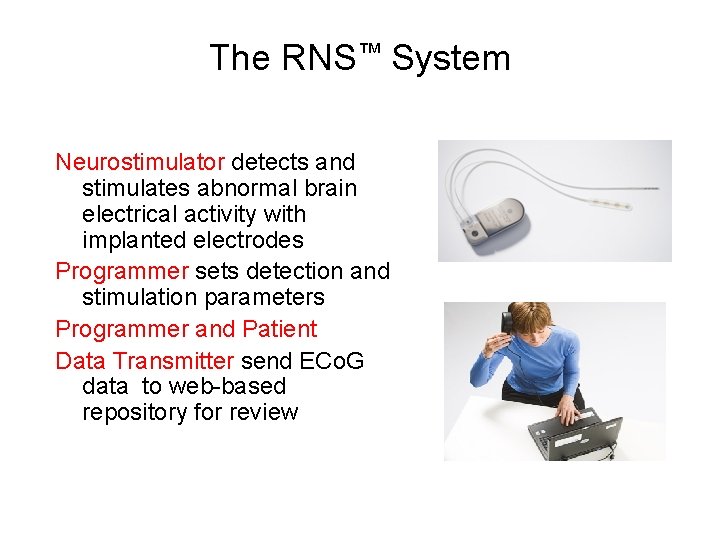
The RNS™ System • Cranially implanted battery powered responsive neurostimulator • Connected to 2 leads (depth and/or subdural) with 4 electrode contacts each

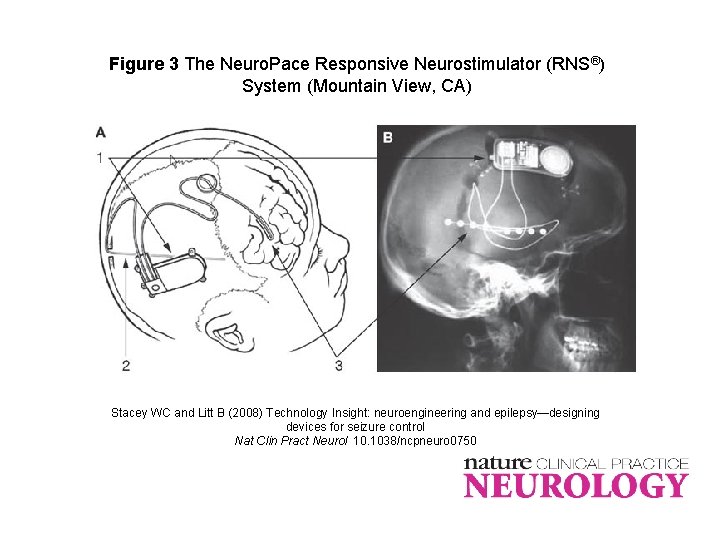
Figure 3 The Neuro. Pace Responsive Neurostimulator (RNS®) System (Mountain View, CA) Stacey WC and Litt B (2008) Technology Insight: neuroengineering and epilepsy—designing devices for seizure control Nat Clin Pract Neurol 10. 1038/ncpneuro 0750

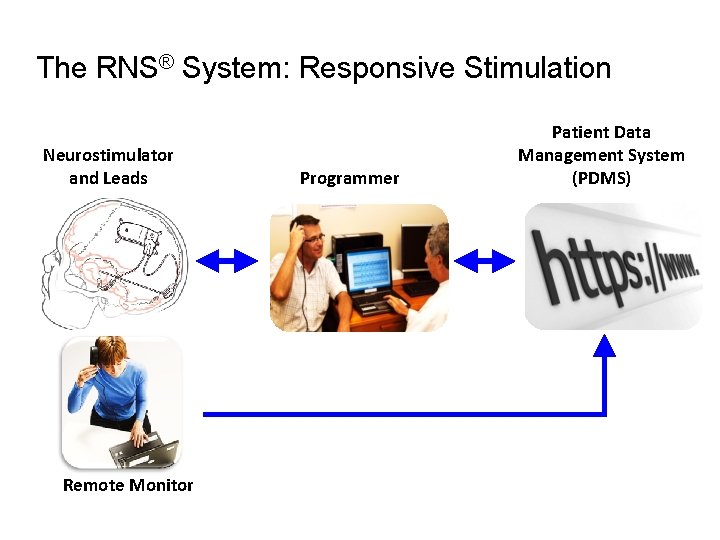
The RNS® System: Responsive Stimulation Neurostimulator and Leads Remote Monitor Programmer Patient Data Management System (PDMS)

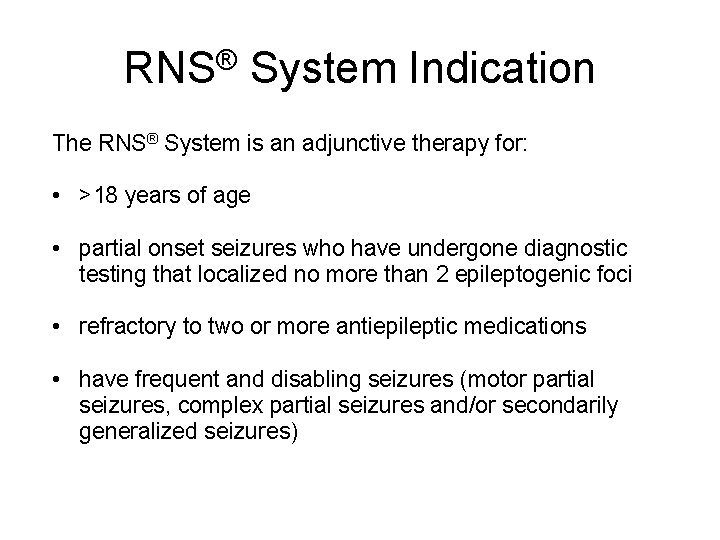
RNS® System Indication The RNS® System is an adjunctive therapy for: • >18 years of age • partial onset seizures who have undergone diagnostic testing that localized no more than 2 epileptogenic foci • refractory to two or more antiepileptic medications • have frequent and disabling seizures (motor partial seizures, complex partial seizures and/or secondarily generalized seizures)

The RNS™ System Neurostimulator detects and stimulates abnormal brain electrical activity with implanted electrodes Programmer sets detection and stimulation parameters Programmer and Patient Data Transmitter send ECo. G data to web-based repository for review

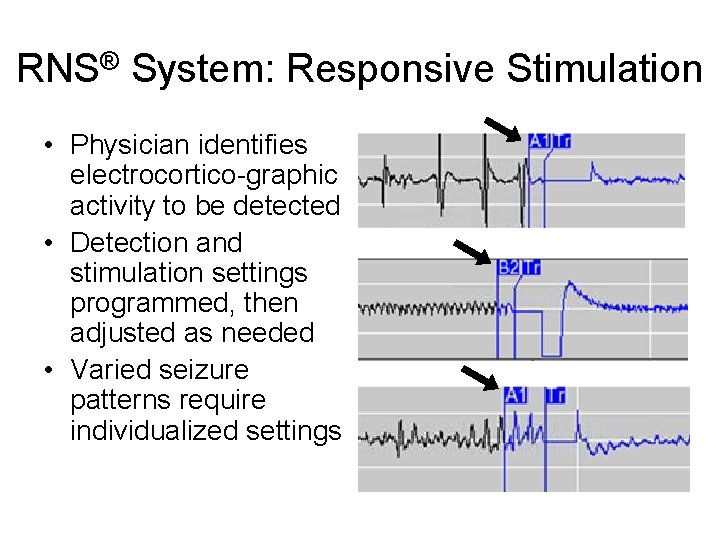
RNS® System: Responsive Stimulation • Physician identifies electrocortico-graphic activity to be detected • Detection and stimulation settings programmed, then adjusted as needed • Varied seizure patterns require individualized settings

Patient Data Management System Caution: Investigational. Device. Limited by US Law to Investigational Use Only. Caution: investigational device. Limited by US law to investigational use only.

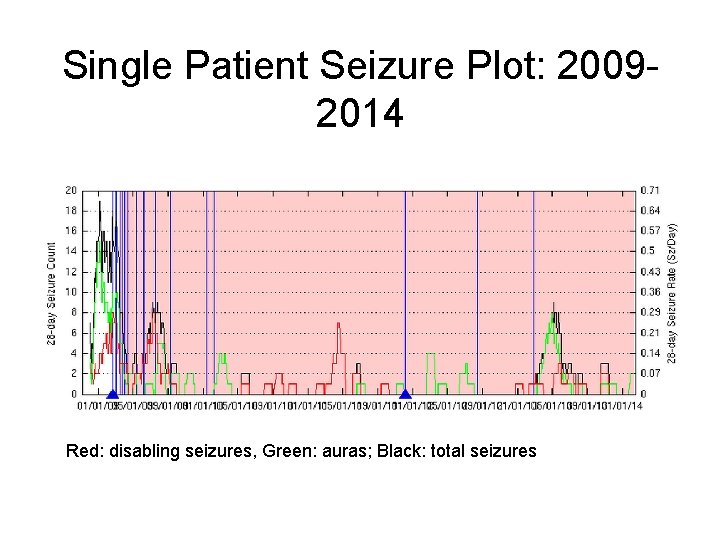
Single Patient Seizure Plot: 20092014 Red: disabling seizures, Green: auras; Black: total seizures

The Neuro. Pace Responsive Neurostimulator (RNS®) System Trial • 191 patients; 32 centers • Randomized to Treatment vs. Sham group for 3 months • Need to know localization of epileptic brain tissue 1 - 2 foci • 3 or more disabling seizures per month

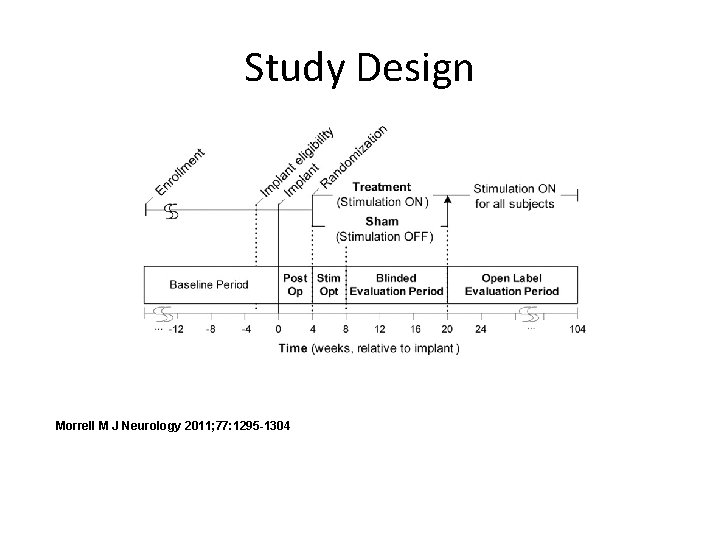
Study Design Morrell M J Neurology 2011; 77: 1295 -1304

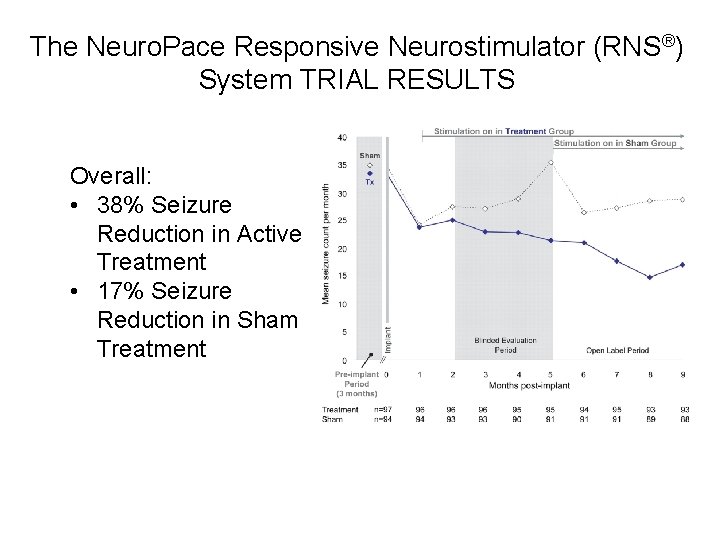
The Neuro. Pace Responsive Neurostimulator (RNS®) System TRIAL RESULTS Overall: • 38% Seizure Reduction in Active Treatment • 17% Seizure Reduction in Sham Treatment

Other outcomes • Improved quality of life in treated group • No difference in memory function between treated and sham groups

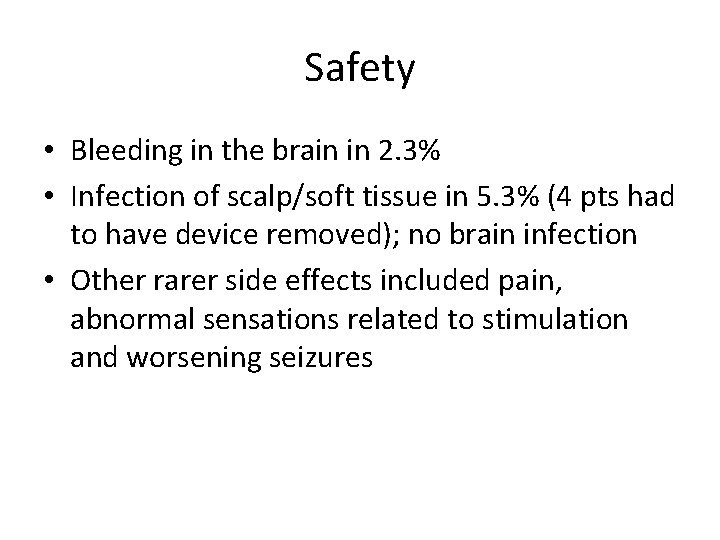
Safety • Bleeding in the brain in 2. 3% • Infection of scalp/soft tissue in 5. 3% (4 pts had to have device removed); no brain infection • Other rarer side effects included pain, abnormal sensations related to stimulation and worsening seizures

Two‐year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: Final results of the RNS System Pivotal trial At the end of 2 years of treatment, 9% were seizure free during the last 3 mo - Those with 1 focus were more likely to be seizure-free ~7% had worsening of seizures Epilepsia Volume 55, Issue 3, pages 432 -441, 22 FEB 2014 DOI: 10. 1111/epi. 12534 http: //onlinelibrary. wiley. com/doi/10. 1111/epi. 12534/full#epi 12534 -fig-0005 Many had improvement in cognitive functioning

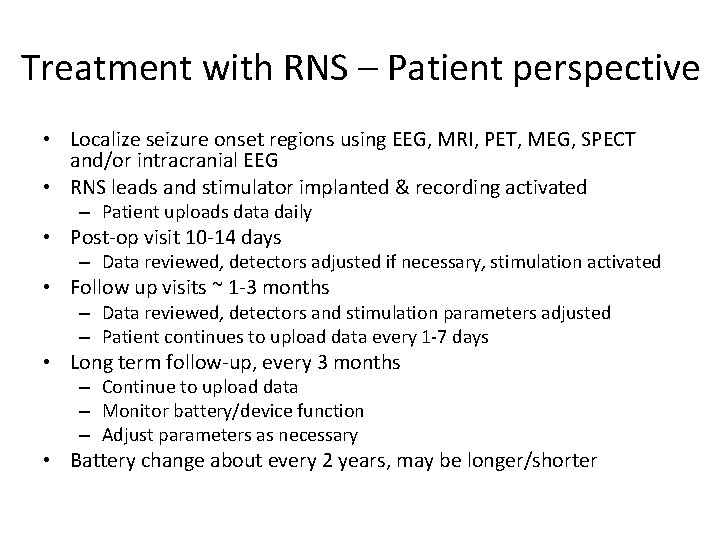
Treatment with RNS – Patient perspective • Localize seizure onset regions using EEG, MRI, PET, MEG, SPECT and/or intracranial EEG • RNS leads and stimulator implanted & recording activated – Patient uploads data daily • Post-op visit 10 -14 days – Data reviewed, detectors adjusted if necessary, stimulation activated • Follow up visits ~ 1 -3 months – Data reviewed, detectors and stimulation parameters adjusted – Patient continues to upload data every 1 -7 days • Long term follow-up, every 3 months – Continue to upload data – Monitor battery/device function – Adjust parameters as necessary • Battery change about every 2 years, may be longer/shorter
 Slidetodoc.com
Slidetodoc.com Canto rallegratevi nel signore
Canto rallegratevi nel signore Rns típusai
Rns típusai Blomman för dagen drog
Blomman för dagen drog Steg för steg rita
Steg för steg rita Redogör för vad psykologi är
Redogör för vad psykologi är En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Bästa kameran för astrofoto
Bästa kameran för astrofoto Mat för unga idrottare
Mat för unga idrottare Lek med former i förskolan
Lek med former i förskolan Etik och ledarskap etisk kod för chefer
Etik och ledarskap etisk kod för chefer Svenskt ramverk för digital samverkan
Svenskt ramverk för digital samverkan Dikt i bunden form
Dikt i bunden form Antika plagg
Antika plagg Vilken grundregel finns det för tronföljden i sverige?
Vilken grundregel finns det för tronföljden i sverige? Ministerstyre för och nackdelar
Ministerstyre för och nackdelar Big brother rösta
Big brother rösta