Response Evaluation Criteria in Solid Tumors RECIST 1









- Slides: 16

Response Evaluation Criteria in Solid Tumors RECIST 1. 1 Criteria Handout

Basic Paradigm �Assess at baseline � Look for measurable lesions � Select target and non-target lesions � Measure target lesions � Follow-up evaluation � Measure target lesions � Assess non-target lesions and look for new lesions � Calculate timepoint response

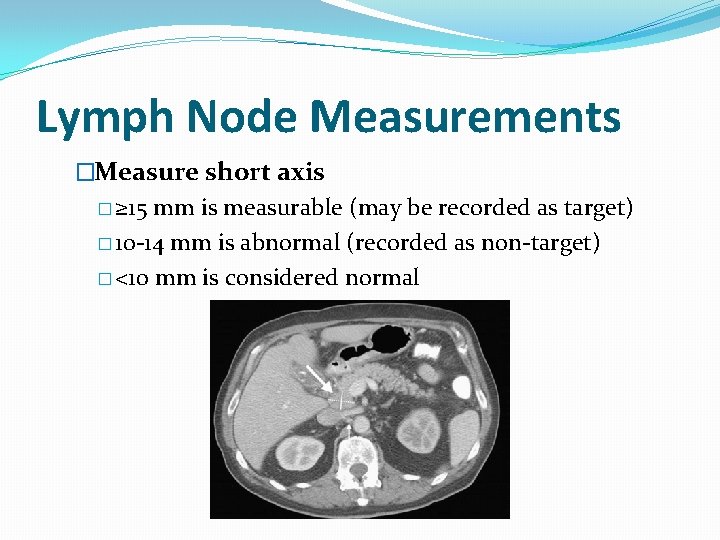
Measurable Lesions �Tumor ≥ 10 mm in longest diameter (LD) on an axial image on CT or MRI �Lymph nodes ≥ 15 mm in short axis on CT �Lymph node measurements: specific instructions: � short axis ≥ 15 mm is measurable � 10 -14 mm is abnormal (recorded as non-target) � <10 mm is considered normal �Ultrasound cannot be used to measure lesions

Non-Measurable Lesions �All other definite tumor lesions � Masses <10 mm � Lymph nodes 10 -14 mm in short axis � Leptomeningeal disease � Ascites, pleural or pericardial effusion � Inflammatory breast disease � Lymphangitic involvement of skin or lung

Special Lesion Types �Bone Lesions �Bone lesions with identifiable soft tissue components seen on CT or MR can be measurable if the soft tissue component meets the definition above � Blastic bone lesions are unmeasurable

Special Lesion Types �Cystic Lesions � Simple cysts are not included as lesions � Cystic metastases may be selected, but prefer to use non-cystic lesions as “target”

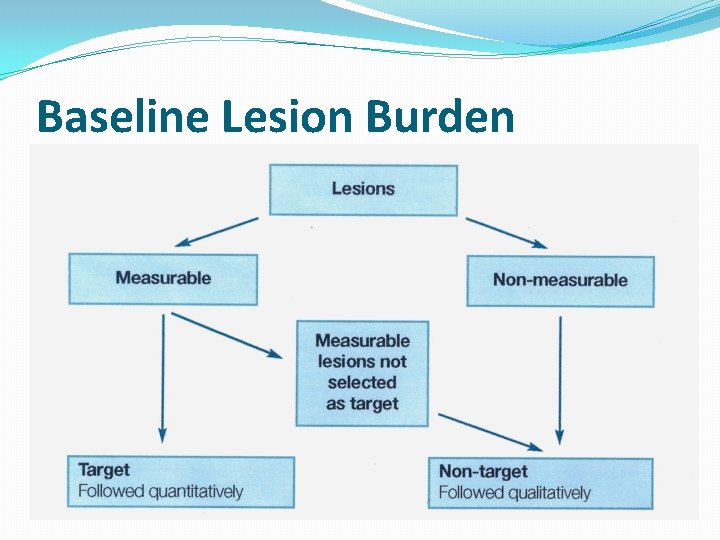
Baseline Lesion Burden

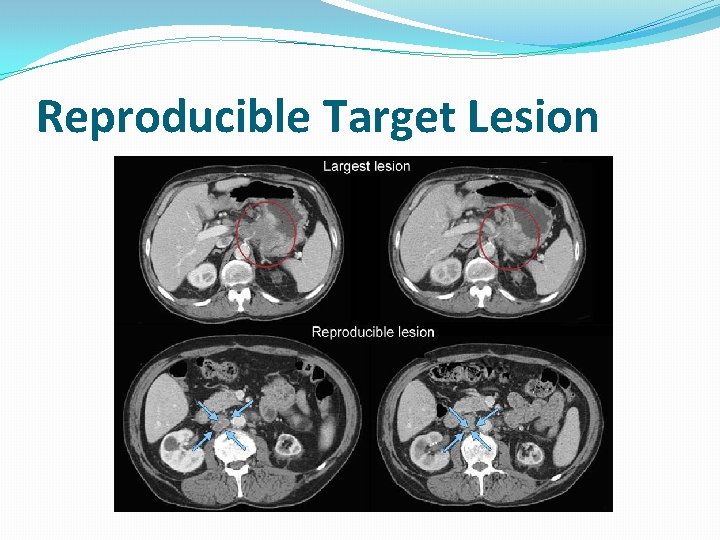
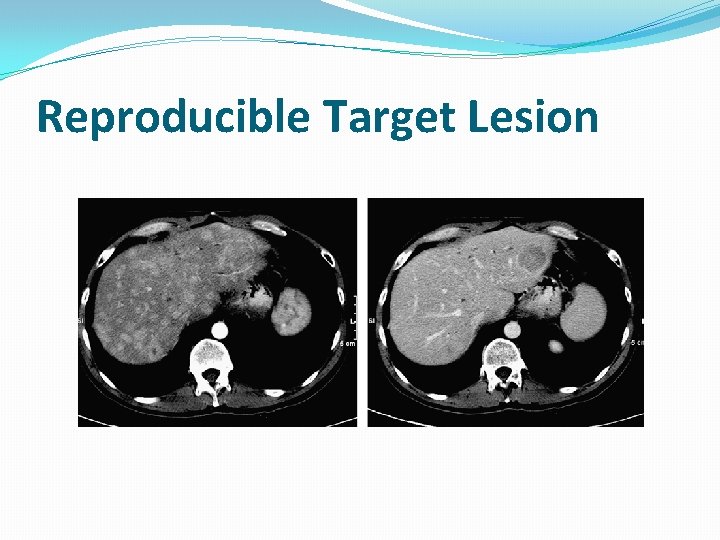
Target Lesions �Record a maximum of five (5) target lesions in total �Up to two (2) per organ �Any combination of organ masses or lymph nodes, but representative of all involved organs � Select largest reproducibly measurable lesions � If the largest lesion cannot be measured reproducibly, select the next largest lesion which can be

Reproducible Target Lesion

Reproducible Target Lesion

Lymph Node Measurements �Measure short axis � ≥ 15 mm is measurable (may be recorded as target) � 10 -14 mm is abnormal (recorded as non-target) � <10 mm is considered normal

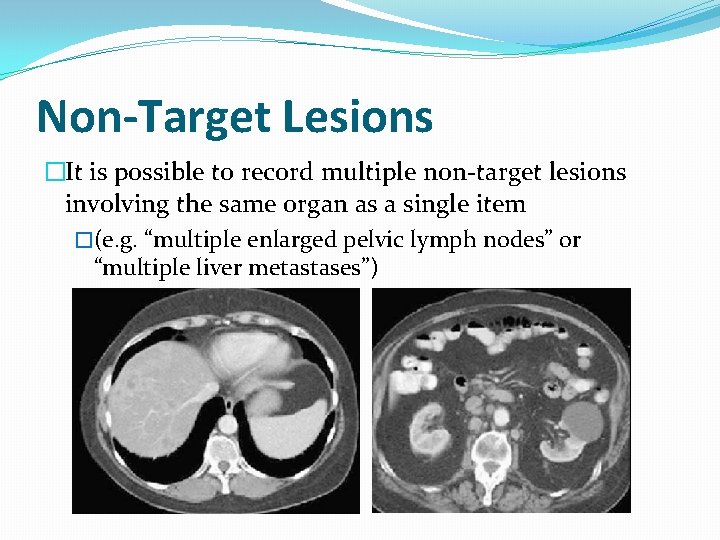
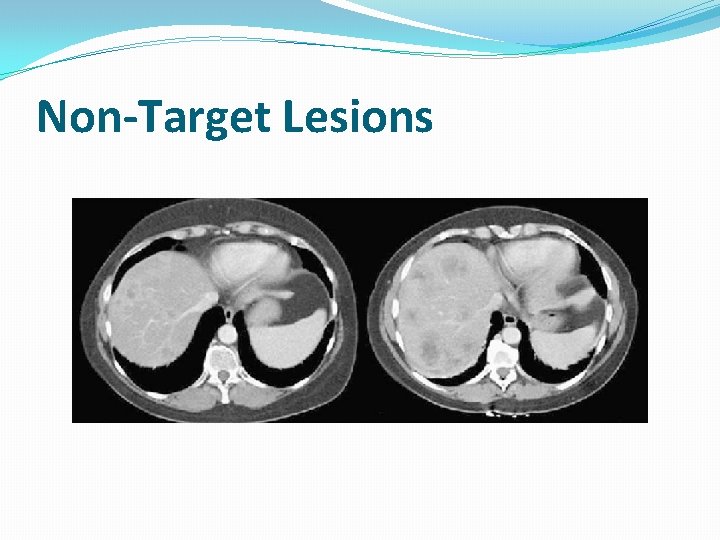
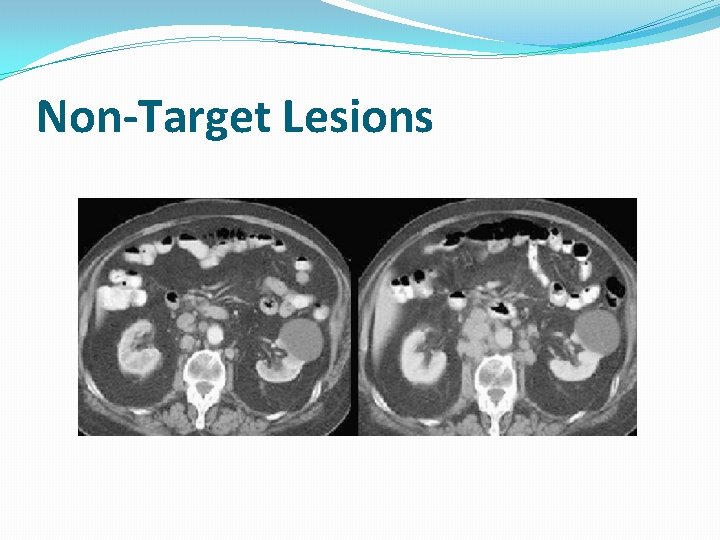
Non-Target Lesions �It is possible to record multiple non-target lesions involving the same organ as a single item �(e. g. “multiple enlarged pelvic lymph nodes” or “multiple liver metastases”)

Evaluation on Follow-up �Measure previously chosen target lesions �Even if they are no longer the largest �If a target lesion fragments into multiple smaller lesions, the LDs of all fragmented portions are measured �If target lesions coalesce, the LD of the resulting coalescent lesion is measured �Evaluate all previously identified non-target lesions �Look for new definite cancer lesions

Non-Target Lesions

Non-Target Lesions

New Lesions �Should be unequivocal and not attributable to differences in scanning technique or findings which may not be a tumor �Does not have to meet criteria to be “measurable” �Lesions identified in anatomic locations not scanned at baseline are considered new �New lesions on US should be confirmed on CT/MRI