Respiratory System Structure and Function of Heamoglobin Core

Respiratory System: Structure and Function of Heamoglobin Core Course No. ZOOA – P 4 T, Group-A, Topic No. 3
Why we need a Str. Like Hemoglobin to transport and store oxygen? ? ? Oxygen is poorly soluble in aqueous solution. Diffusion of O 2 through tissues is ineffective over distances >few mm. Transition metals such as iron, copper etc. In multicellular organisms, especially those in which iron, in its O 2 carrying capacity, must be transported over large distances.
Haemoglobin §Haemoglobin (MW 64, 500, Hb) is roughly spherical §Contain two alpha chains (141 amino acids residues) & two beta chains (146 amino acids residues) §Three-dimensional structures of the 2 types of subunits are very similar. Their structure are very similar to that of myoglobin. §The quaternary structure of Hb features strong interaction b/w unlike subunits. §The α 1β 1 interface (and its α 2β 2 counterparts) involves more than 30 residues. §Mild treatment of Hb with urea tends to disassemble the tetramer into αβ dimer.
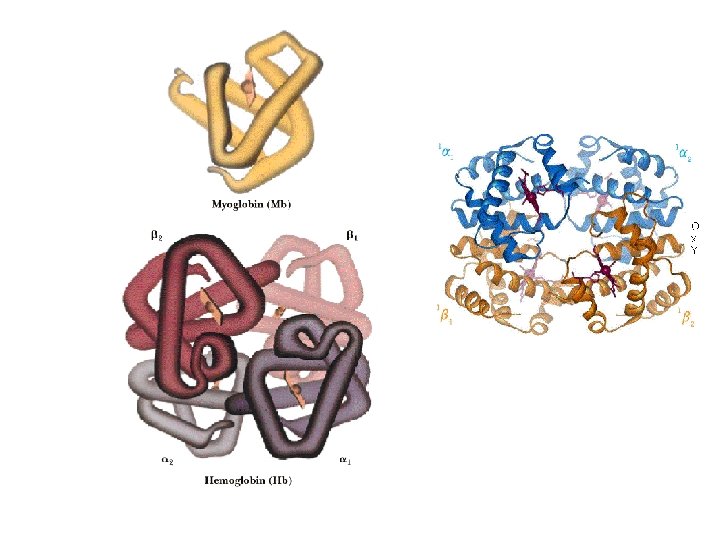
Hb transports oxygen in blood • Normal Human RBCs are small (6 -9µm in diameter), biconcave disks; produced from precursor stem cells (hemocytoblasts). • RBCs are unable to reproduce and, in humans, survive for 120 days only. • Main function is to carry Hb, which is dissolved in the cytosol at a very high conc. (=34% by wt. ). • Arterial blood passing from lung to the peripheral tissues, Hb is about 96% saturated with O 2. Venous blood, Hb is only about 64% saturated.
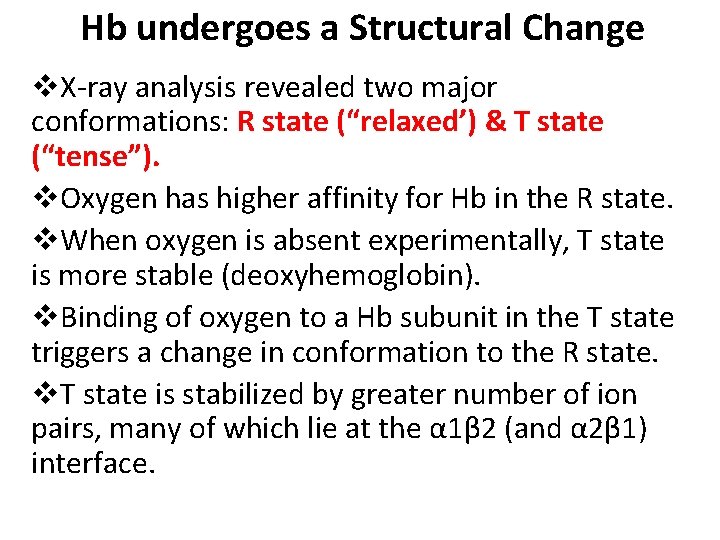
Hb undergoes a Structural Change v. X-ray analysis revealed two major conformations: R state (“relaxed’) & T state (“tense”). v. Oxygen has higher affinity for Hb in the R state. v. When oxygen is absent experimentally, T state is more stable (deoxyhemoglobin). v. Binding of oxygen to a Hb subunit in the T state triggers a change in conformation to the R state. v. T state is stabilized by greater number of ion pairs, many of which lie at the α 1β 2 (and α 2β 1) interface.
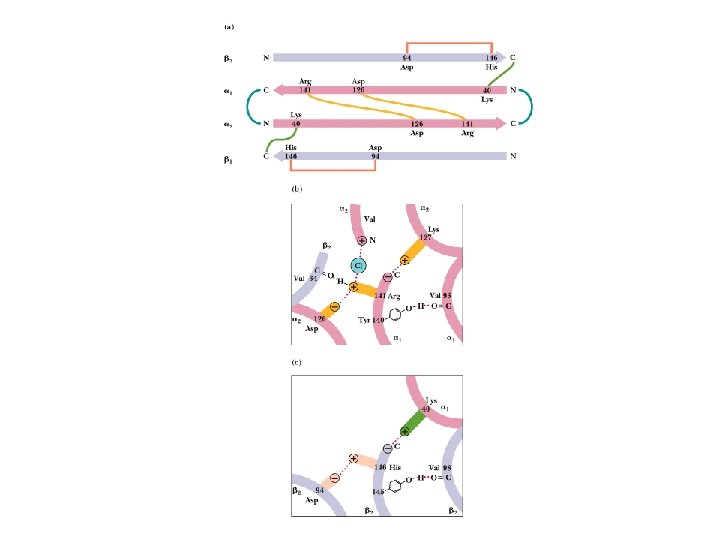
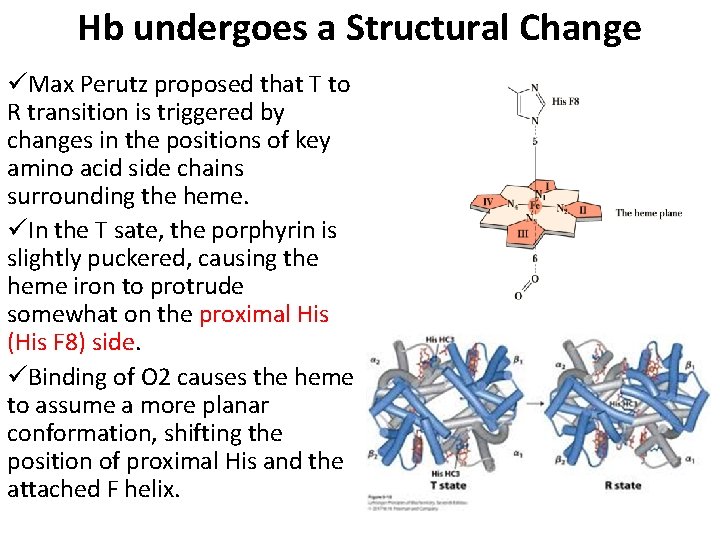
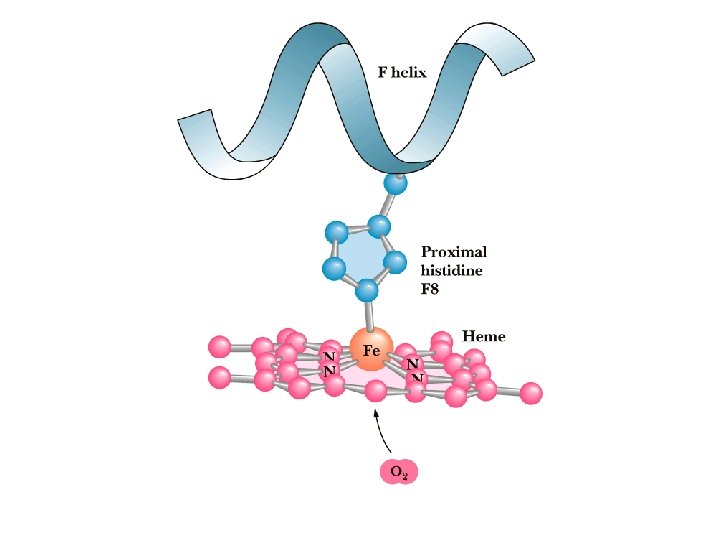
Hb undergoes a Structural Change üMax Perutz proposed that T to R transition is triggered by changes in the positions of key amino acid side chains surrounding the heme. üIn the T sate, the porphyrin is slightly puckered, causing the heme iron to protrude somewhat on the proximal His (His F 8) side. üBinding of O 2 causes the heme to assume a more planar conformation, shifting the position of proximal His and the attached F helix.
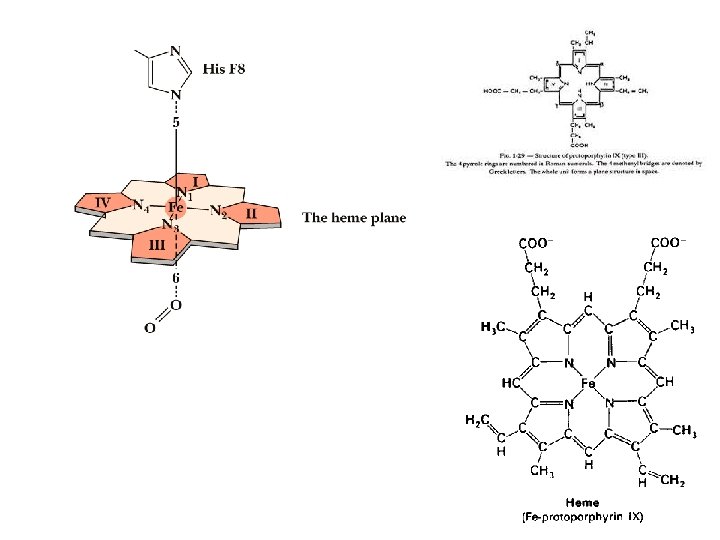
Oxygen can bind to a Heme Prosthetic Group • In multicellular organisms, especially those in which iron, in its O 2 -carrying capacity, must be transported over large distances. • Iron is often incorporated into a protein-bound prosthetic group called heme (haem). • Heme consists of a complex organic ring structure, protoporphyrin, to which is bound a single iron atom (Fe 2+). • The iron atom has 6 coordination bonds, 4 to nitrogen atoms that are part of the flat porphyrin ring sysytem and 2 perpendicular to the porphyrin.
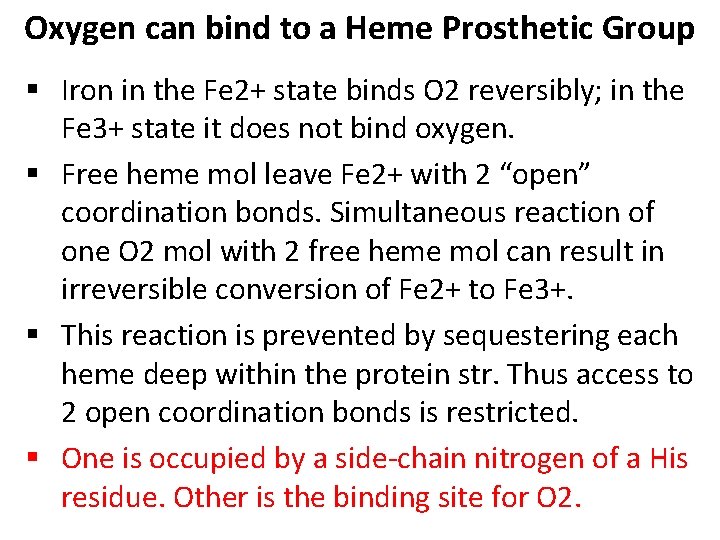
Oxygen can bind to a Heme Prosthetic Group § Iron in the Fe 2+ state binds O 2 reversibly; in the Fe 3+ state it does not bind oxygen. § Free heme mol leave Fe 2+ with 2 “open” coordination bonds. Simultaneous reaction of one O 2 mol with 2 free heme mol can result in irreversible conversion of Fe 2+ to Fe 3+. § This reaction is prevented by sequestering each heme deep within the protein str. Thus access to 2 open coordination bonds is restricted. § One is occupied by a side-chain nitrogen of a His residue. Other is the binding site for O 2.
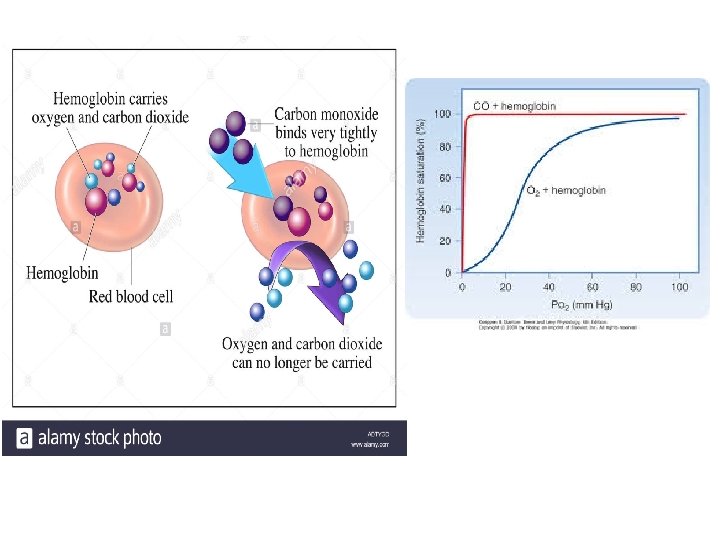
CO poisoning ØSome small molecules, such as CO and NO, coordinate to heme iron with greater affinity than does O 2. ØWhen a mol. of CO is bound to heme, O 2 is excluded, which is why CO is highly toxic to aerobic organisms. ØCO has an approx. 250 -fold greater affinity for Hb than does O 2. ØRelatively low levels of CO can have substantial and tragic effects. ØWhen CO combines with Hb, called carboxyhemoglobin (COHb).
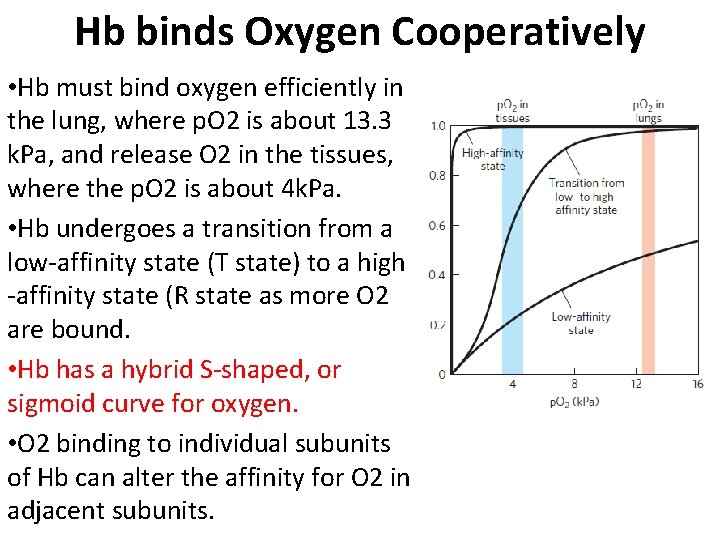
Hb binds Oxygen Cooperatively • Hb must bind oxygen efficiently in the lung, where p. O 2 is about 13. 3 k. Pa, and release O 2 in the tissues, where the p. O 2 is about 4 k. Pa. • Hb undergoes a transition from a low-affinity state (T state) to a high -affinity state (R state as more O 2 are bound. • Hb has a hybrid S-shaped, or sigmoid curve for oxygen. • O 2 binding to individual subunits of Hb can alter the affinity for O 2 in adjacent subunits.

Hb behaves like an Allosteric Protein ØAllosteric (allos=other, stereos=solid/shape) proteins are those having “other shapes”, or conformations, induced by the binding of ligands referred to as modulators. ØModulators may be either inhibitors or activators. ØWhen the normal ligand & modulator are same Homotropic interaction ØWhen modulator is a mol. other than normal ligand – Heterotropic interaction
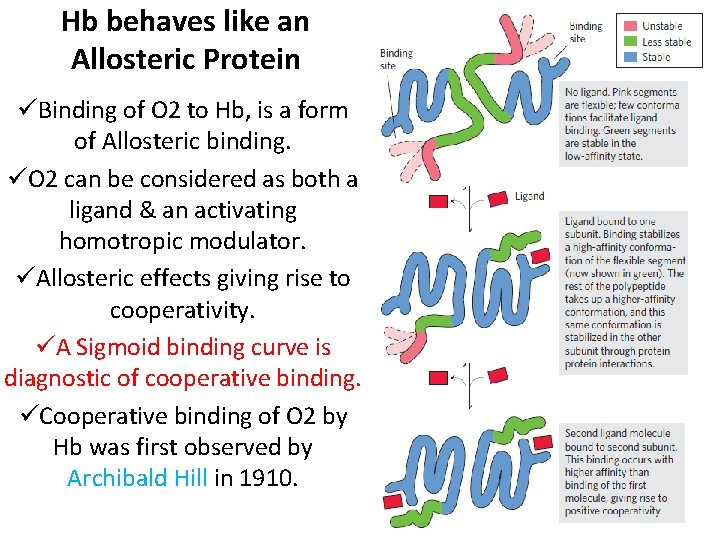
Hb behaves like an Allosteric Protein üBinding of O 2 to Hb, is a form of Allosteric binding. üO 2 can be considered as both a ligand & an activating homotropic modulator. üAllosteric effects giving rise to cooperativity. üA Sigmoid binding curve is diagnostic of cooperative binding. üCooperative binding of O 2 by Hb was first observed by Archibald Hill in 1910.
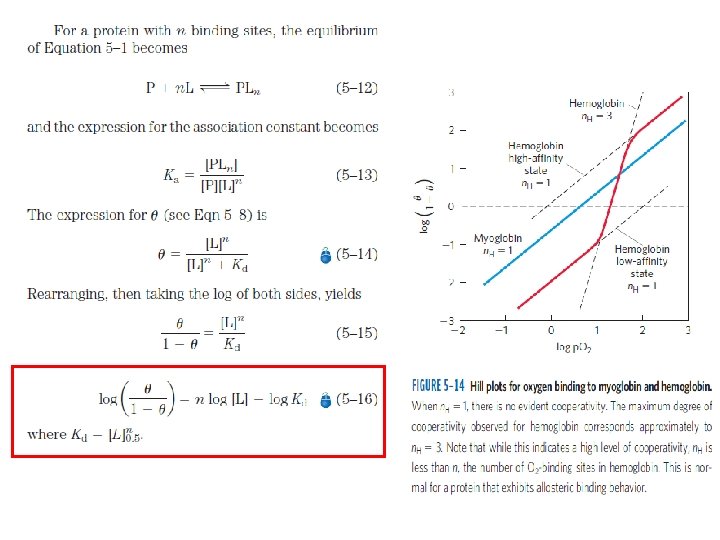

Model for Allosteric Behaviour Two models for the cooperative binding of ligands to proteins with multiple binding sites --1. MWC model or the Concerted model 2. The Sequential model
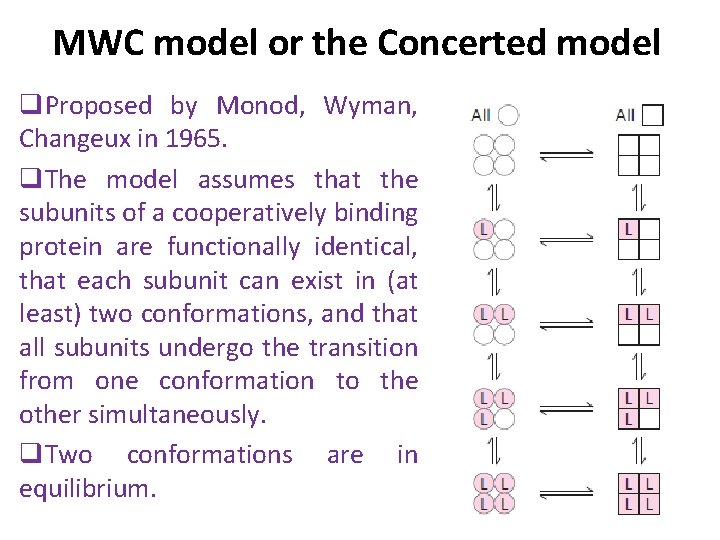
MWC model or the Concerted model q. Proposed by Monod, Wyman, Changeux in 1965. q. The model assumes that the subunits of a cooperatively binding protein are functionally identical, that each subunit can exist in (at least) two conformations, and that all subunits undergo the transition from one conformation to the other simultaneously. q. Two conformations are in equilibrium.
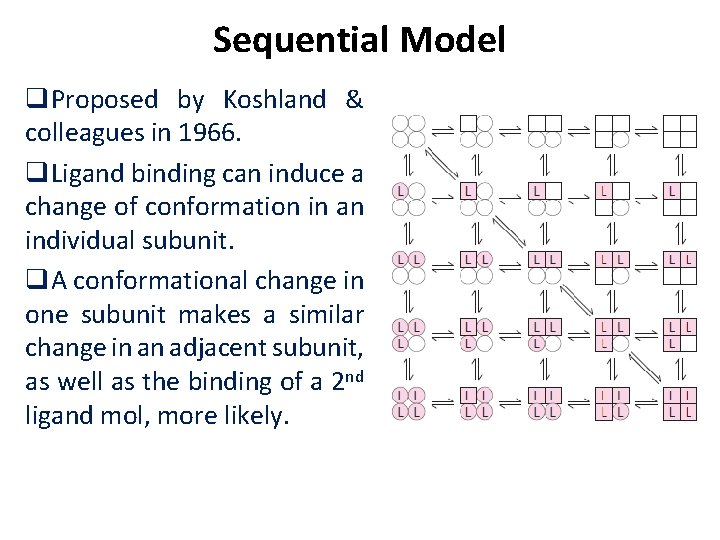
Sequential Model q. Proposed by Koshland & colleagues in 1966. q. Ligand binding can induce a change of conformation in an individual subunit. q. A conformational change in one subunit makes a similar change in an adjacent subunit, as well as the binding of a 2 nd ligand mol, more likely.
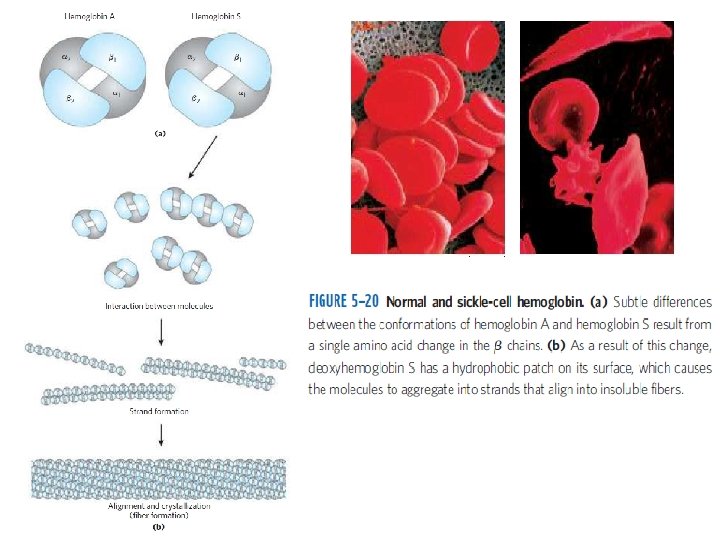

References: 1. Nelson, Cox and Lehninger (2017). Principles of Biochemistry. 7 th Ed. Freeman and Co. 2. Guyton & Hall (2016). Textbook of Medical Physiology. Second South Asia Ed. ELSEVIER
- Slides: 23