RESPIRATORY SYSTEM 3 GAS EXCHANGE AND TRANSPORT Gas








- Slides: 42

RESPIRATORY SYSTEM (3): GAS EXCHANGE AND TRANSPORT

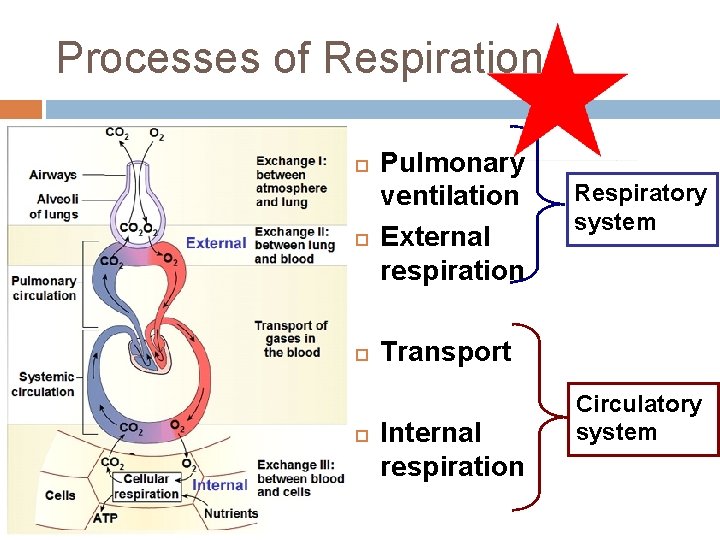
Gas Exchange: The diffusion of O 2 and CO 2 moving in opposite directions. Occurs in two places: -alveoli and capillaries: external respiration -capillaries and cells – internal respiration

Goals/Objectives To apply gas law relationships between partial pressure, solubility, and concentration to gas exchange. Describe how oxygen is transported in blood, Describe carbon dioxide transport in blood

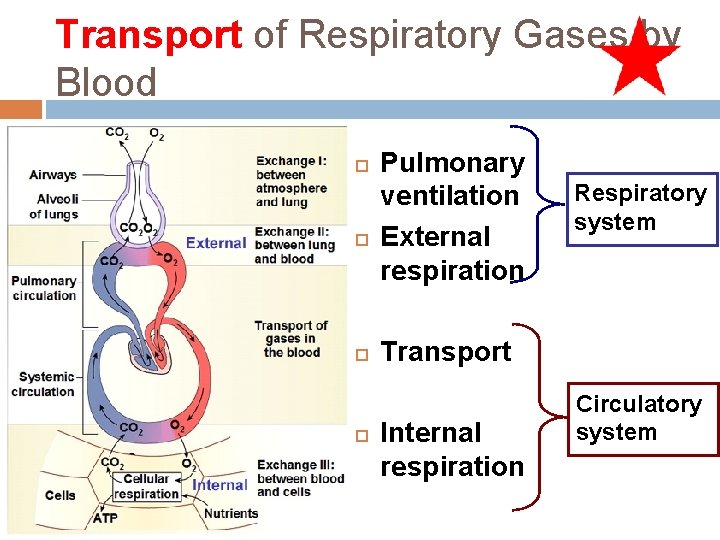
Processes of Respiration Pulmonary ventilation External respiration Respiratory system Transport Internal respiration Circulatory system

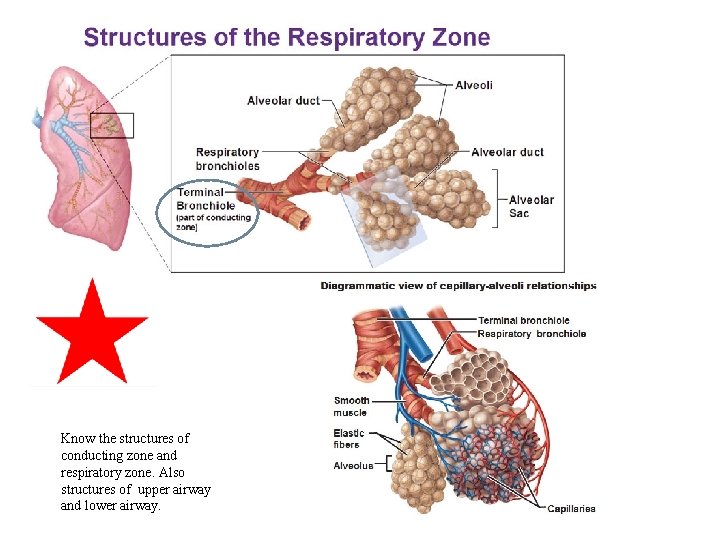
Know the structures of conducting zone and respiratory zone. Also structures of upper airway and lower airway.


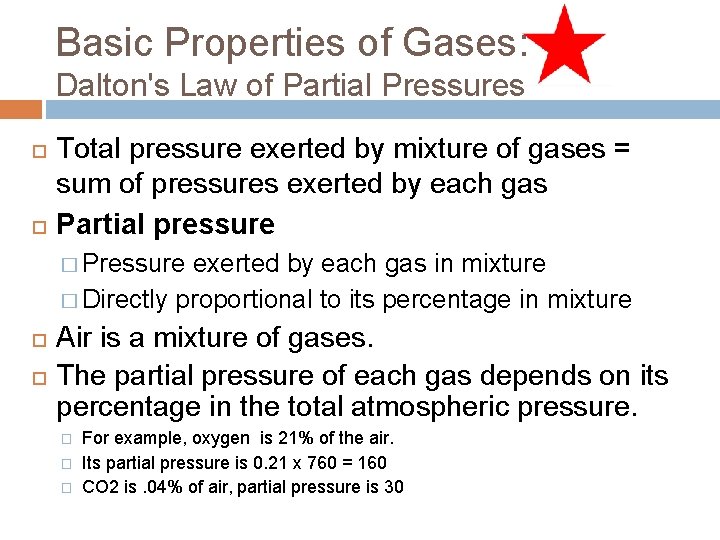
Basic Properties of Gases: Dalton's Law of Partial Pressures Total pressure exerted by mixture of gases = sum of pressures exerted by each gas Partial pressure � Pressure exerted by each gas in mixture � Directly proportional to its percentage in mixture Air is a mixture of gases. The partial pressure of each gas depends on its percentage in the total atmospheric pressure. � � � For example, oxygen is 21% of the air. Its partial pressure is 0. 21 x 760 = 160 CO 2 is. 04% of air, partial pressure is 30

Basic Properties of Gases: Henry's Law Gas mixtures in contact with liquid (blood is mostly water) � Each gas dissolves in proportion to its partial pressure � At equilibrium, partial pressures in the two phases (gas and liquid) will be equal Amount of each gas that will dissolve depends on � Solubility–CO 2 20 times more soluble in water than O 2 � Partial pressure of the gas

Gas exchange occurs by partial pressure gradients. The exchange of O 2 and CO 2 as the pulmonary and tissue capillaries is by simple diffusion. A partial pressure gradient is established when there are two partial pressures for a gas in different regions of the body. For example: the partial pressure of O 2 is greater in the alveoli (e. g. , 100) diffuses down its partial pressure gradient towards into the blood of the pulmonary capillaries where the pressure is 40

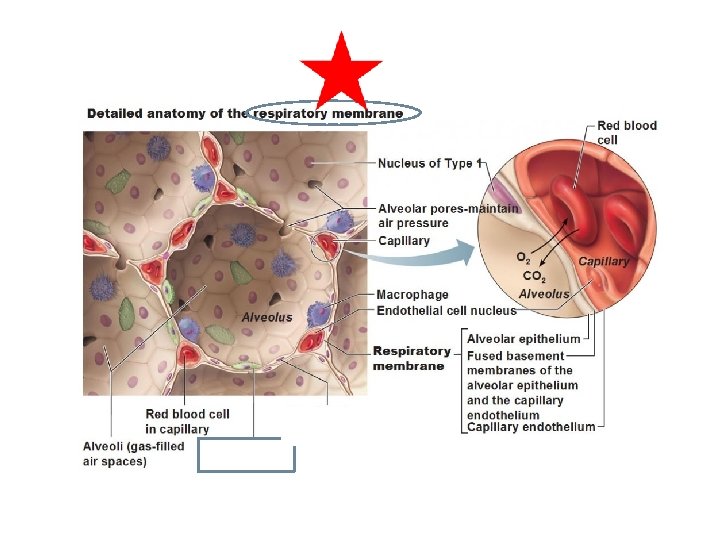
External Respiration- O 2 diffuses into capillaries, CO 2 diffuses into alveoli Three factors influence external respiration: 1) Surface area and structure of the respiratory membrane. 2) Partial pressure gradients ( differences in partial pressures of O 2 and CO 2 between alveoli and capillaries). 3) Ventilation-perfusion coupling: matching alveolar air flow to capillary blood flow.

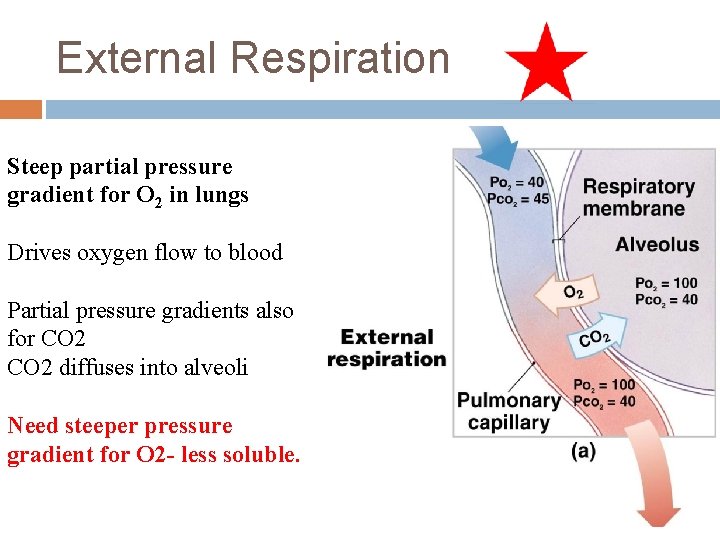
External Respiration Steep partial pressure gradient for O 2 in lungs Drives oxygen flow to blood Partial pressure gradients also for CO 2 diffuses into alveoli Need steeper pressure gradient for O 2 - less soluble.

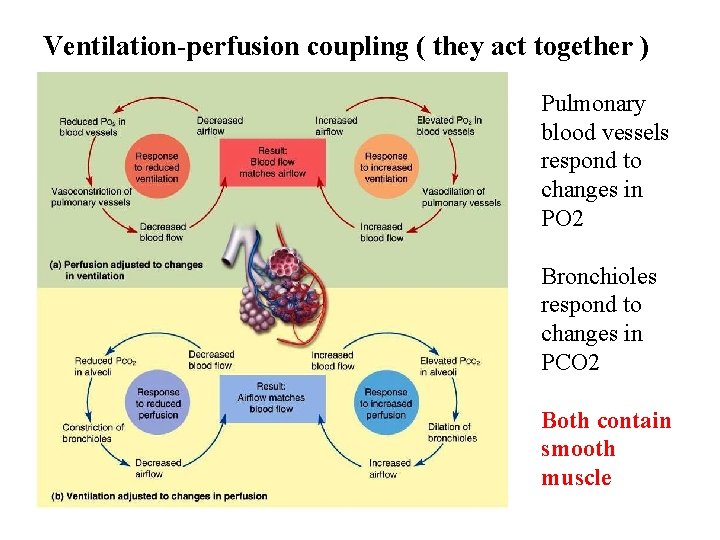
Ventilation-perfusion coupling ( they act together ) Pulmonary blood vessels respond to changes in PO 2 Bronchioles respond to changes in PCO 2 Both contain smooth muscle

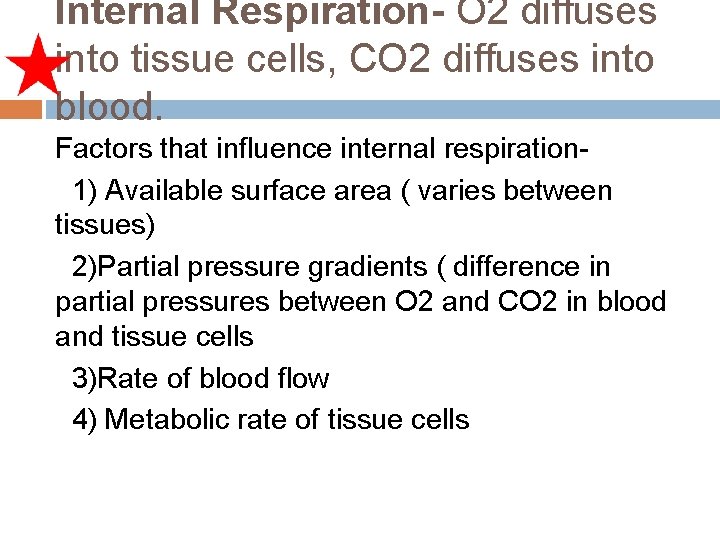
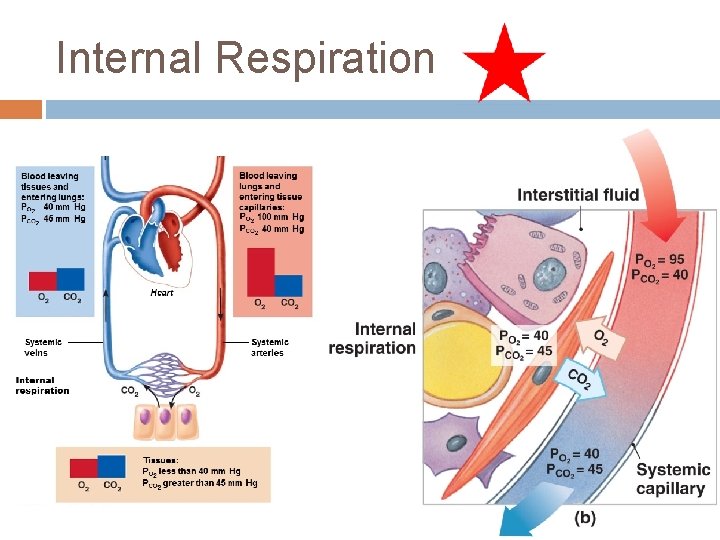
Internal Respiration- O 2 diffuses into tissue cells, CO 2 diffuses into blood. Factors that influence internal respiration 1) Available surface area ( varies between tissues) 2)Partial pressure gradients ( difference in partial pressures between O 2 and CO 2 in blood and tissue cells 3)Rate of blood flow 4) Metabolic rate of tissue cells

Internal Respiration

Transport of Respiratory Gases by Blood Pulmonary ventilation External respiration Respiratory system Transport Internal respiration Circulatory system

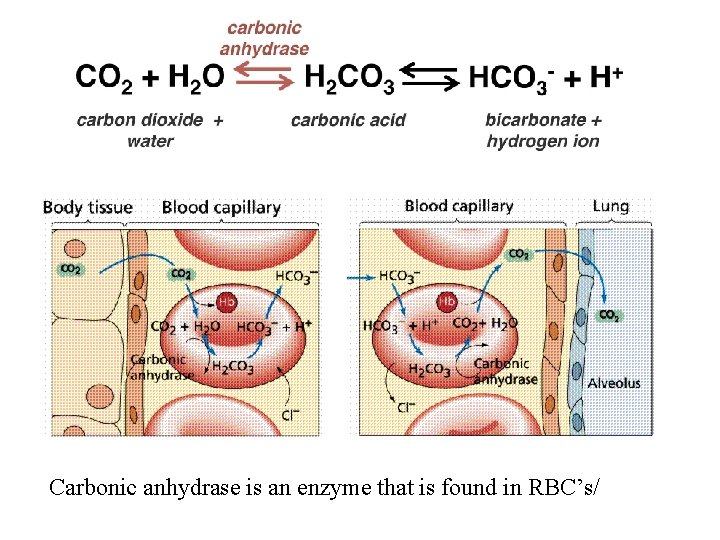
Gas Transport - The blood carries CO 2 and O 2 between the lungs and the tissues. These gases are transported in different forms: Dissolved in the plasma Chemically bound to hemoglobin Or as a different molecule: CO 2 is transported as bicarbonate ions (HCO 3–)

Goals/Objectives Understand Dalton’s law of partial pressures and Henry’s law Relate Dalton’s law and Henry’s laws to events of external and internal respiration Describe how oxygen is transported in blood, Describe carbon dioxide transport in blood

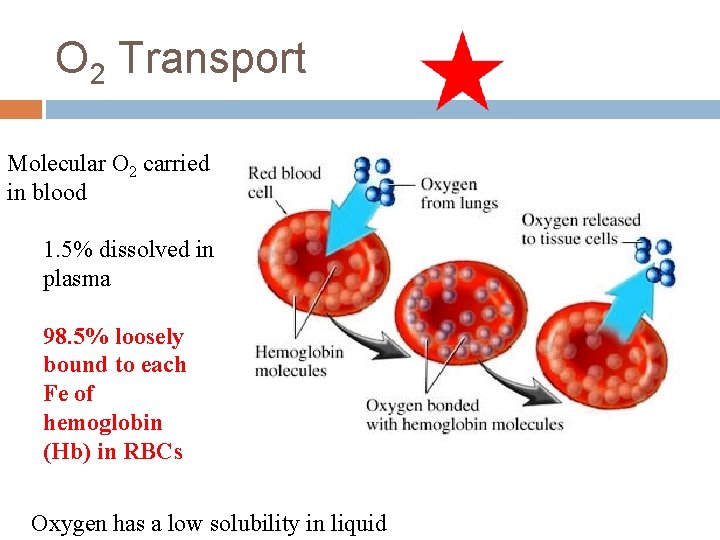
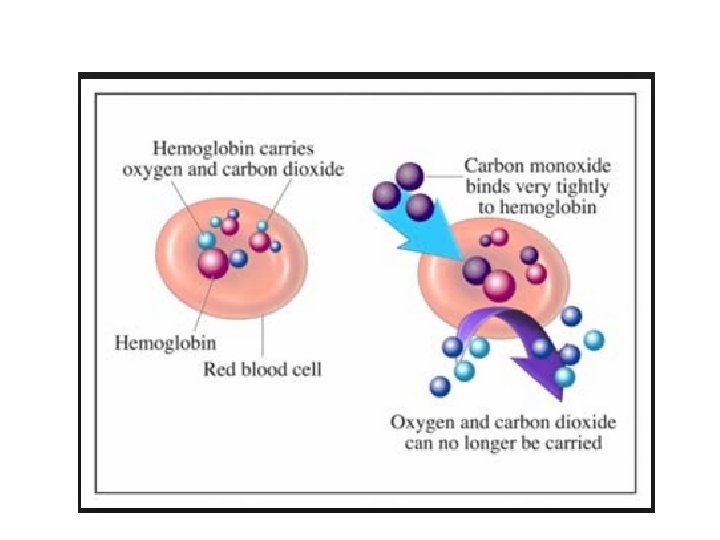
O 2 Transport Molecular O 2 carried in blood 1. 5% dissolved in plasma 98. 5% loosely bound to each Fe of hemoglobin (Hb) in RBCs Oxygen has a low solubility in liquid

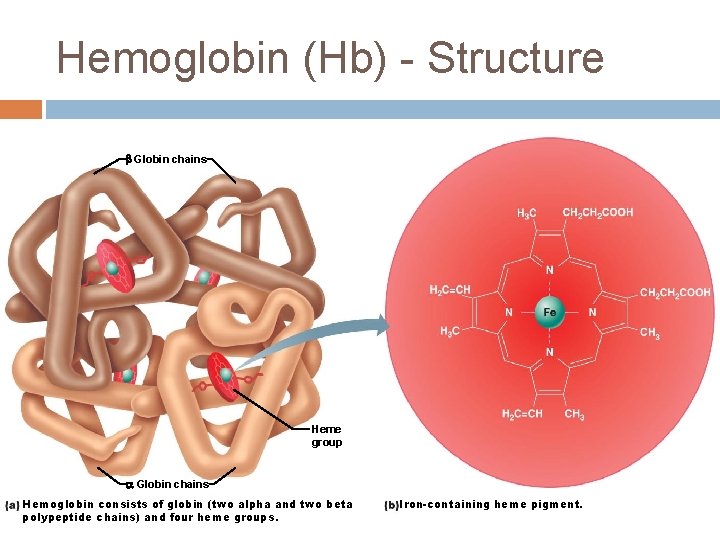
Hemoglobin (Hb) - Structure Globin chains Heme group Globin chains Hemoglobin consists of globin (two alpha and two beta polypeptide chains) and four heme groups. Iron-containing heme pigment.

Hemoglobin molecules can carry up to four molecules of O 2 When all four hemoglobin molecules are attached to O 2 it is said to be 100% saturated. When fewer O 2 molecules are attached, it is partially saturated Oxygen binding occurs in response to the high partial pressure of oxygen in the lungs.

O 2 and Hemoglobin Fully saturated (100%) if all four heme groups carry O 2 Partially saturated when one to three hemes carry O 2

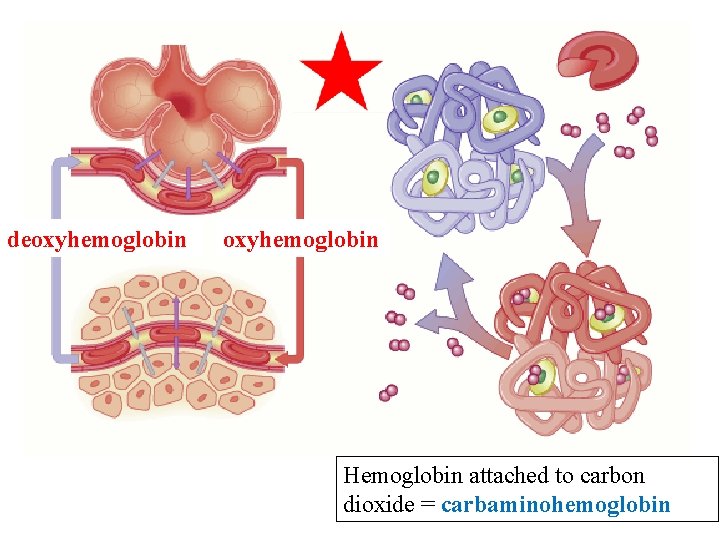
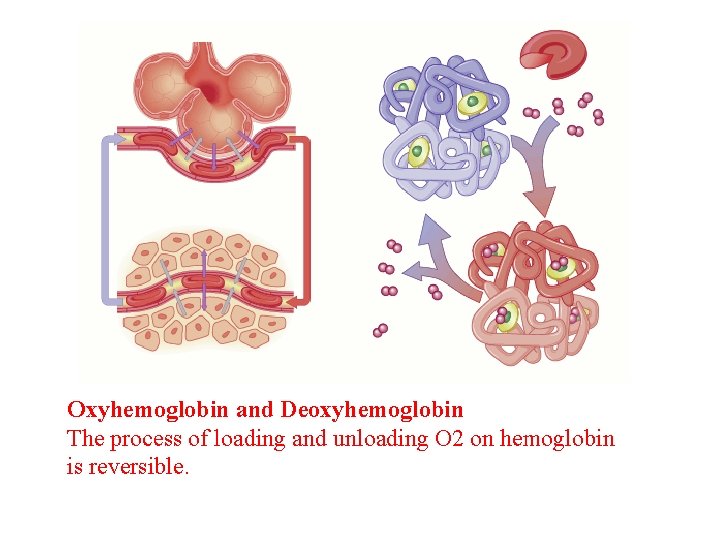
deoxyhemoglobin Hemoglobin attached to carbon dioxide = carbaminohemoglobin

Oxyhemoglobin and Deoxyhemoglobin The process of loading and unloading O 2 on hemoglobin is reversible.

© 2013 Pearson Education, Inc.

Question The maximum molecule(s) of O 2 that can be transported by one hemoglobin molecule is: a) b) c) d) one two three four

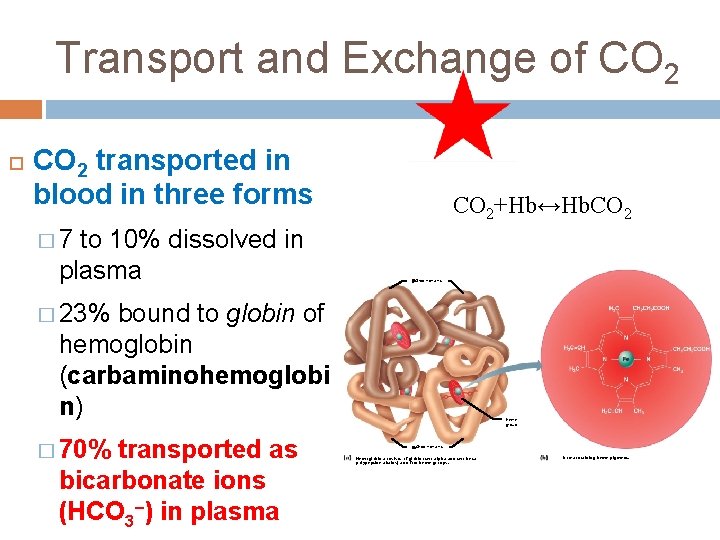
Transport and Exchange of CO 2 transported in blood in three forms to 10% dissolved in plasma CO 2+Hb↔Hb. CO 2 � 7 Globin chains � 23% bound to globin of hemoglobin (carbaminohemoglobi n) � 70% transported as bicarbonate ions (HCO 3–) in plasma Heme group Globin chains Hemoglobin consists of globin (two alpha and two beta polypeptide chains) and four heme groups. Iron-containing heme pigment.

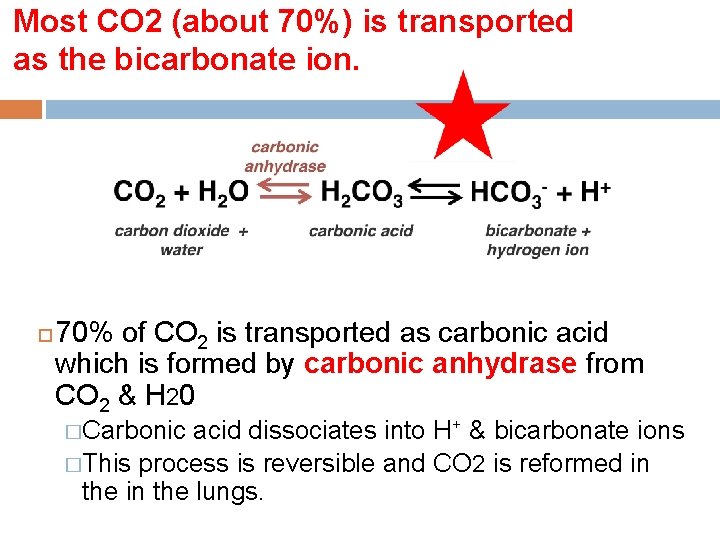
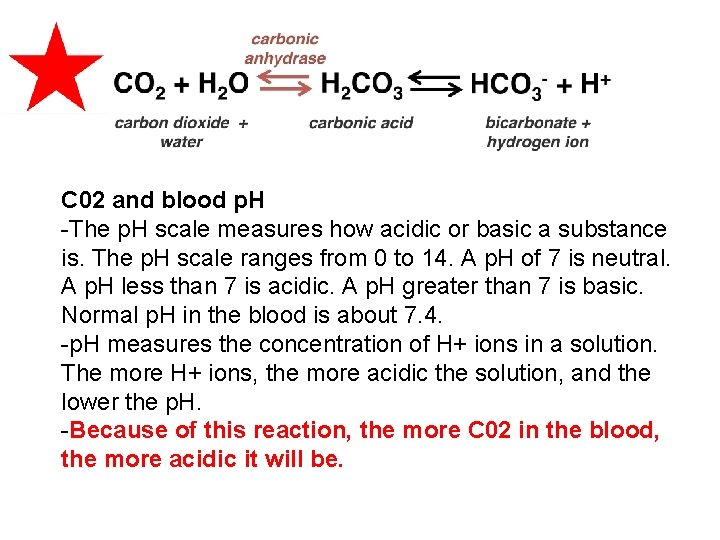
Most CO 2 (about 70%) is transported as the bicarbonate ion. 70% of CO 2 is transported as carbonic acid which is formed by carbonic anhydrase from CO 2 & H 20 �Carbonic acid dissociates into H+ & bicarbonate ions �This process is reversible and CO 2 is reformed in the lungs.

C 02 and blood p. H -The p. H scale measures how acidic or basic a substance is. The p. H scale ranges from 0 to 14. A p. H of 7 is neutral. A p. H less than 7 is acidic. A p. H greater than 7 is basic. Normal p. H in the blood is about 7. 4. -p. H measures the concentration of H+ ions in a solution. The more H+ ions, the more acidic the solution, and the lower the p. H. -Because of this reaction, the more C 02 in the blood, the more acidic it will be.

Carbonic anhydrase is an enzyme that is found in RBC’s/

C 02 and blood p. H CO 2 and p. H have an opposite relationship: The more CO 2, the more acidic, the lower the p. H The less CO 2, the less acidic, the higher the p. H The body aims to maintain a normal p. H ( homeostasis). Hyperventillation ( an increase in breathing rate) will lower blood C 02 and raise the p. H. Hypoventillation ( a decrease in breathing rate ) will raise blood C 02 and lower the p. H.

RESPIRATORY SYSTEM (4): CONTROL AND ADJUSTMENTS

Goals Understand how the respiratory centers control breathing to maintain homeostasis. Understand how p. CO 2, p. H, and p. O 2 affect ventilation. Understand the relationship between breathing and blood p. H. Be able to state examples of other control mechanisms of ventilation.

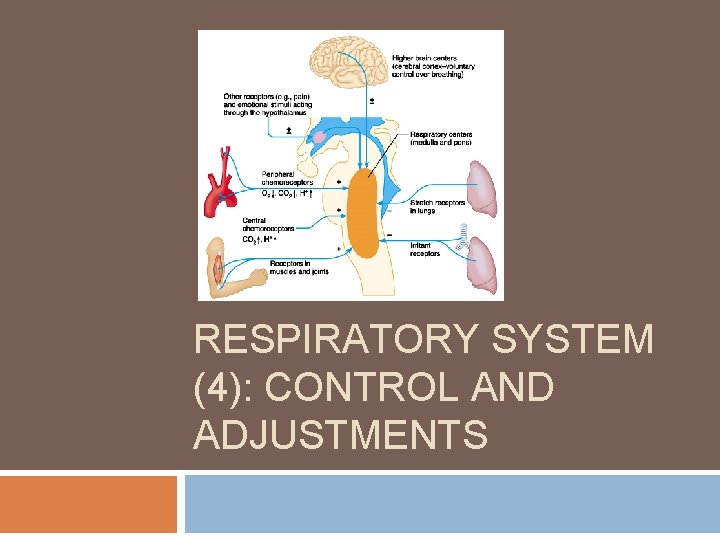
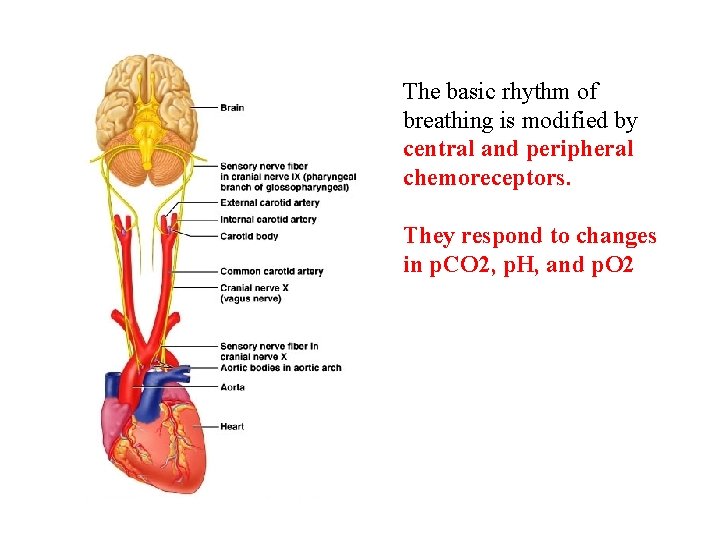
Respiratory System and Homeostasis Sensory receptor – control center- effector Sensory receptors = chemoreceptors that detect arterial blood p. H, p. CO 2, and p. O 2 Control center = respiratory centers in brain Effector = respiratory muscles

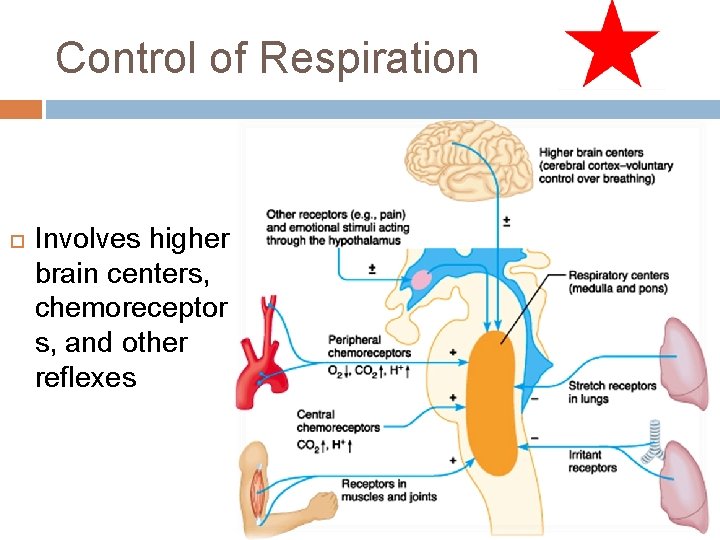
Control of Respiration Involves higher brain centers, chemoreceptor s, and other reflexes

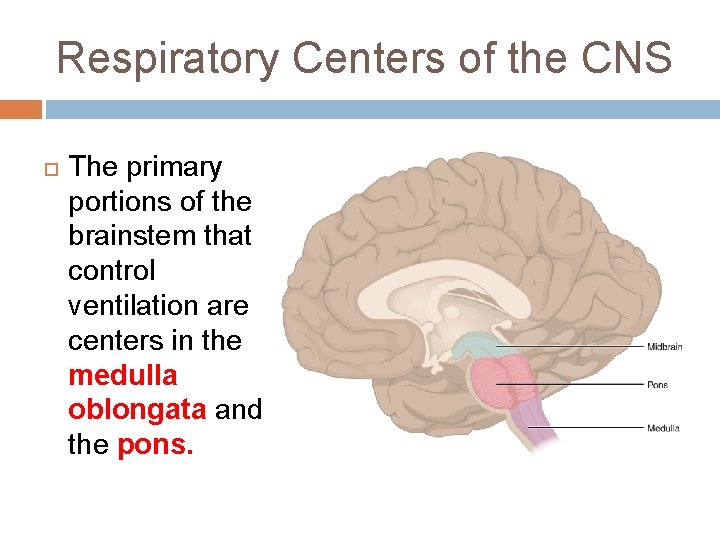
Respiratory Centers of the CNS The primary portions of the brainstem that control ventilation are centers in the medulla oblongata and the pons.

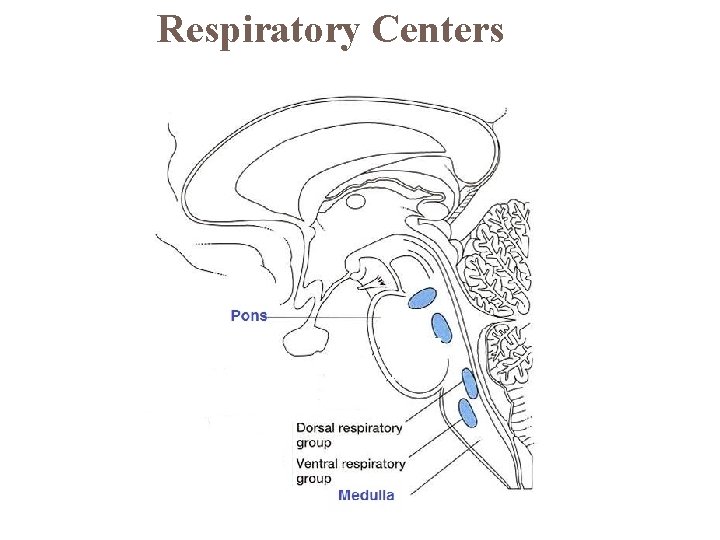
Respiratory Centers

The basic rhythm of breathing is modified by central and peripheral chemoreceptors. They respond to changes in p. CO 2, p. H, and p. O 2

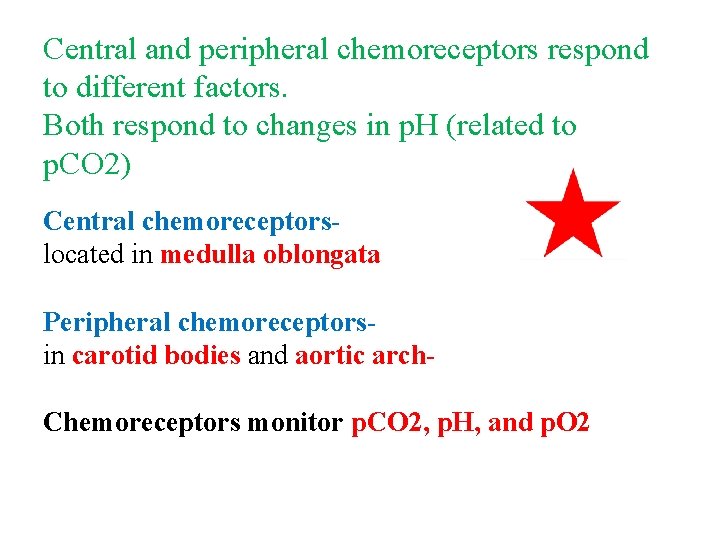
Central and peripheral chemoreceptors respond to different factors. Both respond to changes in p. H (related to p. CO 2) Central chemoreceptorslocated in medulla oblongata Peripheral chemoreceptorsin carotid bodies and aortic arch. Chemoreceptors monitor p. CO 2, p. H, and p. O 2

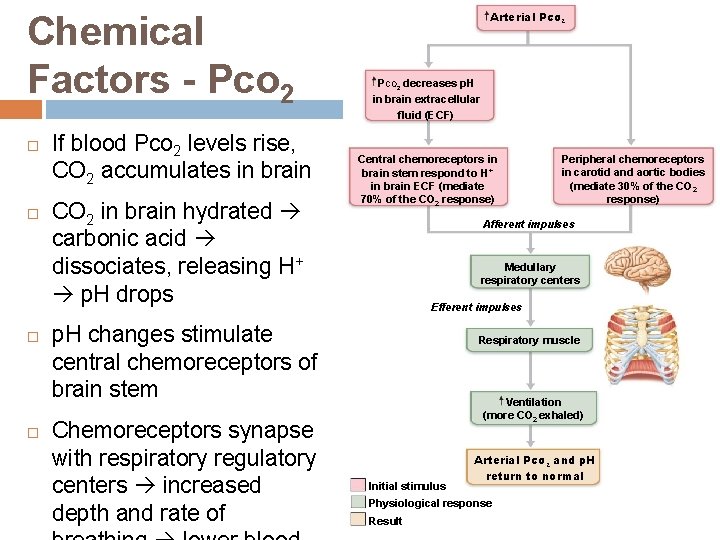
Chemical Factors - Pco 2 If blood Pco 2 levels rise, CO 2 accumulates in brain CO 2 in brain hydrated carbonic acid dissociates, releasing H+ p. H drops Arterial PCO 2 decreases p. H in brain extracellular fluid (ECF) Central chemoreceptors in brain stem respond to H+ in brain ECF (mediate 70% of the CO 2 response) Afferent impulses Medullary respiratory centers Efferent impulses p. H changes stimulate central chemoreceptors of brain stem Chemoreceptors synapse with respiratory regulatory centers increased depth and rate of Peripheral chemoreceptors in carotid and aortic bodies (mediate 30% of the CO 2 response) Respiratory muscle Ventilation (more CO 2 exhaled) Initial stimulus Arterial PCO 2 and p. H return to normal Physiological response Result

Chemical Factors - Arterial p. H • Can modify respiratory rate and rhythm even if CO 2 and O 2 levels normal Respiratory system control centers attempt to raise p. H by increasing respiratory rate and depth. • • Decreased p. H may reflect • CO 2 retention; accumulation of lactic acid

Hyperventilation/Hypoventilation Predict the changes that can occur when someone hyperventilates: (breathes faster and deeper) • p. CO 2 will _increase/decrease_____? • p. H will __ increase/decrease______? • This will signal the chemoreceptors to tell the respiratory muscles to_ breathe faster/deeper or slower/less deep________? Now, predict what happens when someone hypoventilates (breathes slower or less deep)

Question When blood CO 2 levels increase, ______. a) The p. H of the blood drops b) Chemoreceptors detect changes in p. H c) Depth and rate of breathing increases d) All of the above