Respiration as Redox w Respiration is a redox






- Slides: 15

Respiration as Redox w Respiration is a redox process that transfers hydrogen from sugar to oxygen. • Valence electrons of carbon and hydrogen lose potential energy as they shift toward electronegative oxygen. • Released energy is used by cells to produce ATP. • Organic molecules with abundant hydrogen are excellent fuels because they are rich in high-energy electrons. A mole of glucose yields 686 Kcal of heat when burned in air.

Stepwise conversion of Energy • Cellular combustion of glucose is not a single explosive step, as the energy would be difficult to harness. • In the cell, glucose is oxidized gradually in a series of enzyme-controlled steps that occur during glycolysis and the citric acid cycle (Krebs cycle). • Hydrogens stripped from glucose are not transferred directly to oxygen, but are first passed to a special electron acceptor - NAD+ or FAD.

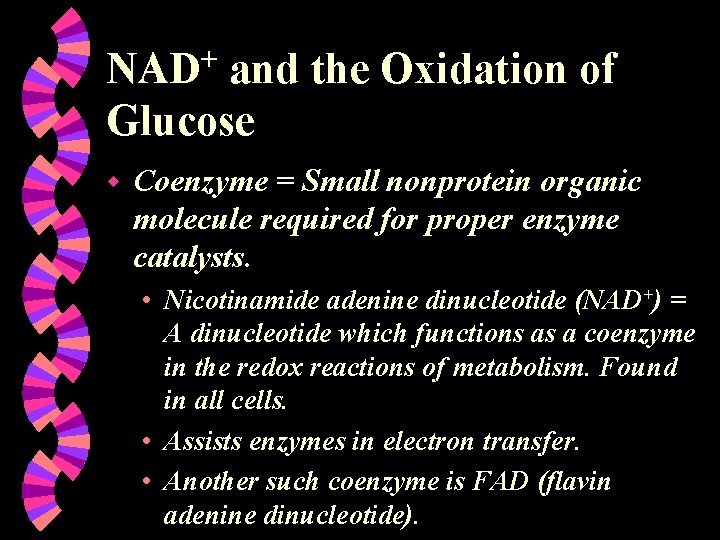
NAD+ and the Oxidation of Glucose w Coenzyme = Small nonprotein organic molecule required for proper enzyme catalysts. • Nicotinamide adenine dinucleotide (NAD+) = A dinucleotide which functions as a coenzyme in the redox reactions of metabolism. Found in all cells. • Assists enzymes in electron transfer. • Another such coenzyme is FAD (flavin adenine dinucleotide).

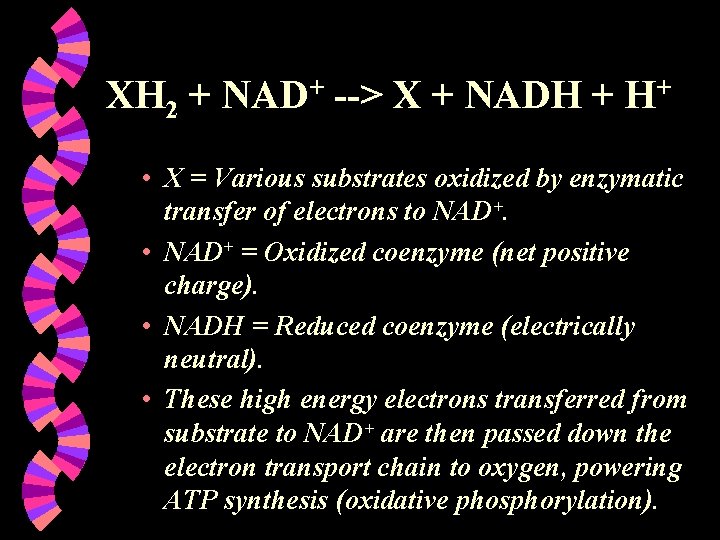
XH 2 + NAD+ --> X + NADH + H+ • X = Various substrates oxidized by enzymatic transfer of electrons to NAD+. • NAD+ = Oxidized coenzyme (net positive charge). • NADH = Reduced coenzyme (electrically neutral). • These high energy electrons transferred from substrate to NAD+ are then passed down the electron transport chain to oxygen, powering ATP synthesis (oxidative phosphorylation).

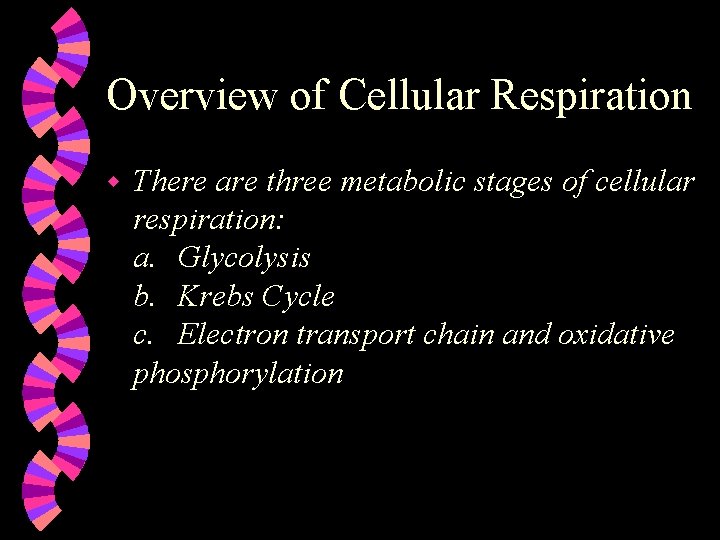
Overview of Cellular Respiration w There are three metabolic stages of cellular respiration: a. Glycolysis b. Krebs Cycle c. Electron transport chain and oxidative phosphorylation

Overview-cont’d. w Glycolysis is a catabolic pathway that: • Occurs in the cytosol. • Partially oxidizes glucose (6 C) into two pyruvic acid (3 C) molecules. w The Krebs Cycle is a catabolic pathway that: • Is located within the mitochondrial matrix. • Completes glucose oxidation by breaking down a pyruvic acid derivative (acetyl Co. A) into carbon dioxide.

Overview-cont’d. w Glycolysis and the Krebs Cycle: • Directly produce a small amount of ATP. • Supply energized electrons that indirectly drive most ATP production by oxidative phosphorylation

Oxidative phosphorylation • accounts for most ATP production during respiration. • includes an electron transport chain made of electron-carrier molecules built into the inner mitochondrial membrane. • Oxygen pulls energized electrons down the electron transport chain to a lower energy state. • This exergonic slide of electrons is coupled to ATP synthesis. • For each molecule of glucose oxidized, the cell makes about 36 to 38 ATP molecules.

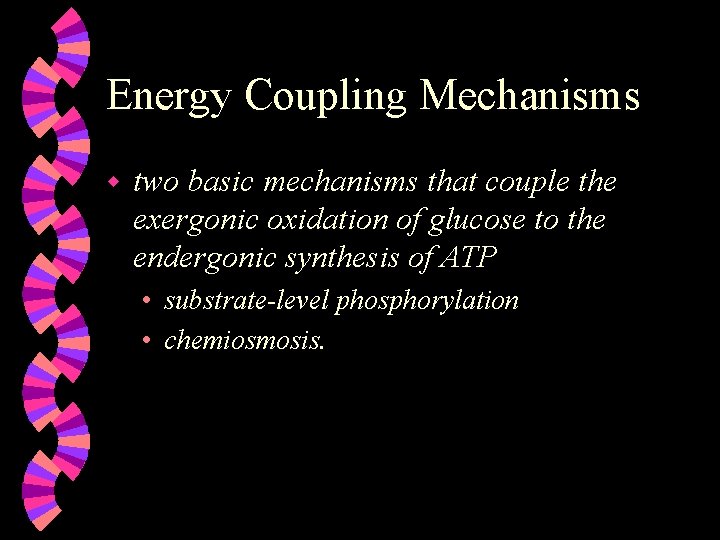
Energy Coupling Mechanisms w two basic mechanisms that couple the exergonic oxidation of glucose to the endergonic synthesis of ATP • substrate-level phosphorylation • chemiosmosis.

Substrate-level phosphorylation the direct enzymatic transfer of phosphate to ADP from an intermediate substrate in catabolism. w Only a small percentage of ATP is produced this way: most ATP is produced by oxidative phosphorylation. w

Chemiosmotic Coupling: The Basic Principle • The mechanism for coupling exergonic electron flow from the oxidation of food to endergonic ATP production is chemiosmosis. • The coupling of exergonic electron flow down an electron transport chain to endergonic ATP production by the creation of an electrochemical proton gradient across a membrane. The proton gradient drives ATP synthesis as protons diffuse back across the membrane.

Chemiosmosis - cont’d. • Proposed by British biochemist, Peter Mitchell (1961). • The term chemiosmosis emphasizes a coupling between (1) chemical reactions and (2) transport processes.

Chemiosmosis - cont’d. w Membrane structure plays a prominent functional role in chemiosmosis: • Integral membrane proteins translocate H+ across a membrane, creating a proton (or p. H) gradient. • The membrane's phospholipid bilayer is impermeable to H+, so it counteracts the tendency for protons to leak back across the membrane by diffusion.

• Transmembrane protein complexes called ATP synthase use the potential energy stored in a proton gradient to make ATP by allowing H+ to diffuse down the gradient, back across the membrane. As protons diffuse through the ATP synthase complex, ATP synthase phosphorylates ADP. • The energy required to create the proton gradient comes from: • Light - during the energy-capturing reactions of photosynthesis. • Oxidation of glucose - during glycolysis and the Krebs Cycle of respiration.

Chemiosmosis w During respiration, chemiosmosis occurs across the inner membrane of the mitochondria. • Using energy from the oxidation of glucose, the electron transport chain translocates H+ from the mitochondrial matrix, across the inner membrane to the intermembrane space. • Cristae or infoldings of the inner mitochondrial membrane, increase the surface area available for chemiosmosis to occur.