Resources and Activities Chemical Equilibrium Textbook chapter 15
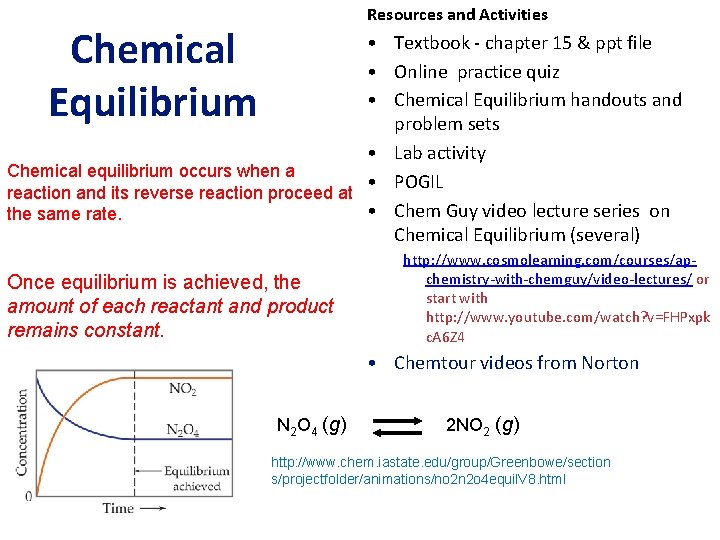
Resources and Activities Chemical Equilibrium • Textbook - chapter 15 & ppt file • Online practice quiz • Chemical Equilibrium handouts and problem sets • Lab activity Chemical equilibrium occurs when a • POGIL reaction and its reverse reaction proceed at • Chem Guy video lecture series on the same rate. Chemical Equilibrium (several) Once equilibrium is achieved, the amount of each reactant and product remains constant. http: //www. cosmolearning. com/courses/apchemistry-with-chemguy/video-lectures/ or start with http: //www. youtube. com/watch? v=FHPxpk c. A 6 Z 4 • Chemtour videos from Norton N 2 O 4 (g) 2 NO 2 (g) http: //www. chem. iastate. edu/group/Greenbowe/section s/projectfolder/animations/no 2 n 2 o 4 equil. V 8. html
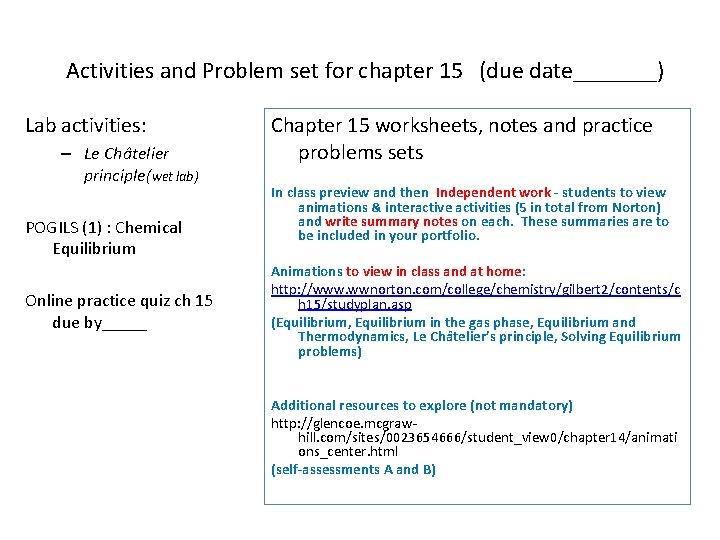
Activities and Problem set for chapter 15 (due date_______) Lab activities: – Le Châtelier principle(wet lab) POGILS (1) : Chemical Equilibrium Online practice quiz ch 15 due by_____ Chapter 15 worksheets, notes and practice problems sets In class preview and then Independent work - students to view animations & interactive activities (5 in total from Norton) and write summary notes on each. These summaries are to be included in your portfolio. Animations to view in class and at home: http: //www. wwnorton. com/college/chemistry/gilbert 2/contents/c h 15/studyplan. asp (Equilibrium, Equilibrium in the gas phase, Equilibrium and Thermodynamics, Le Châtelier’s principle, Solving Equilibrium problems) Additional resources to explore (not mandatory) http: //glencoe. mcgrawhill. com/sites/0023654666/student_view 0/chapter 14/animati ons_center. html (self-assessments A and B)
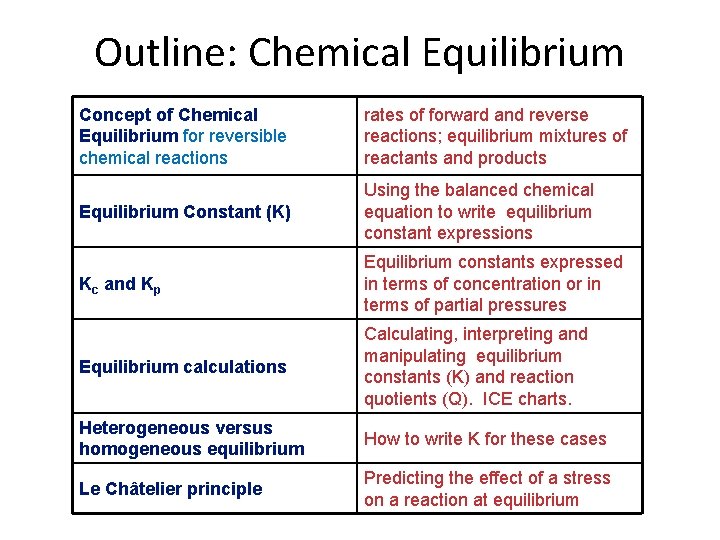
Outline: Chemical Equilibrium Concept of Chemical Equilibrium for reversible chemical reactions rates of forward and reverse reactions; equilibrium mixtures of reactants and products Equilibrium Constant (K) Using the balanced chemical equation to write equilibrium constant expressions Kc and Kp Equilibrium constants expressed in terms of concentration or in terms of partial pressures Equilibrium calculations Calculating, interpreting and manipulating equilibrium constants (K) and reaction quotients (Q). ICE charts. Heterogeneous versus homogeneous equilibrium How to write K for these cases Le Châtelier principle Predicting the effect of a stress on a reaction at equilibrium
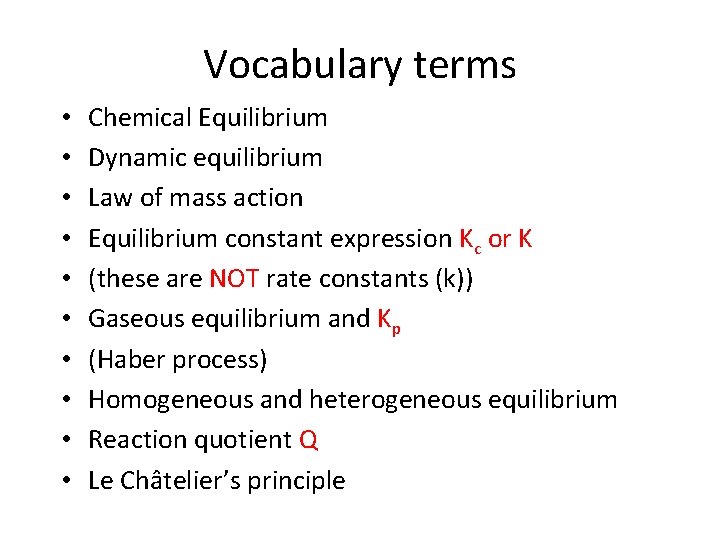
Vocabulary terms • • • Chemical Equilibrium Dynamic equilibrium Law of mass action Equilibrium constant expression Kc or K (these are NOT rate constants (k)) Gaseous equilibrium and Kp (Haber process) Homogeneous and heterogeneous equilibrium Reaction quotient Q Le Châtelier’s principle
- Slides: 4