Resonance Structures By Tina India Shelby Zain Resonance

Resonance Structures By: Tina, India, Shelby, Zain
Resonance Structures Resonance structures are alternative forms of Lewis structures that give and equally accurate description of a single molecule. l Used when there are multiple ways to show bonds in a Lewis structure diagram of a molecule l

Resonance Structures NO 3 - One nitrogen-oxygen double bond, two nitrogen-oxygen single bonds Double headed arrows indicate contributing resonance structures Structures have the same types of bonds and electron positions, but are not identical; position of nitrogen-oxygen double bond makes them different Measurements show all bond lengths are equal because three bonds are intermediate between single and double
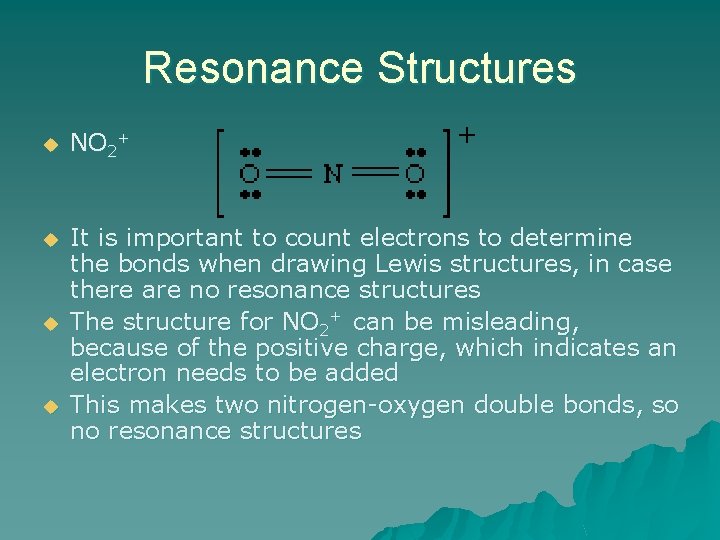
Resonance Structures u NO 2+ u It is important to count electrons to determine the bonds when drawing Lewis structures, in case there are no resonance structures The structure for NO 2+ can be misleading, because of the positive charge, which indicates an electron needs to be added This makes two nitrogen-oxygen double bonds, so no resonance structures u u
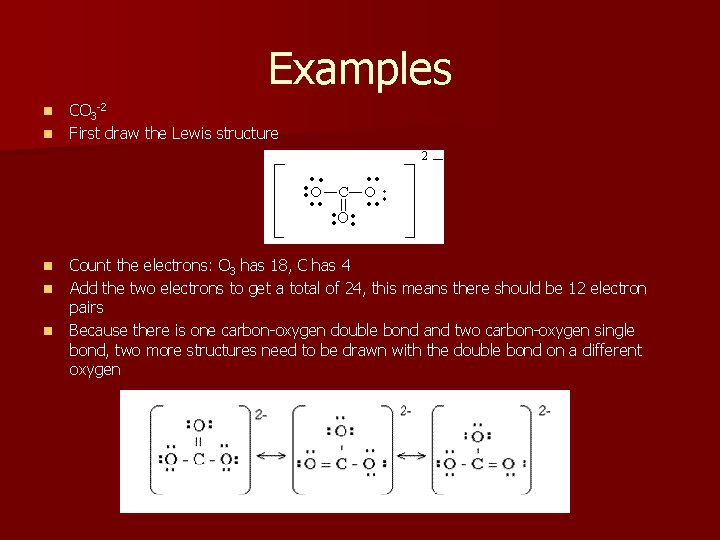
Examples n n n CO 3 -2 First draw the Lewis structure Count the electrons: O 3 has 18, C has 4 Add the two electrons to get a total of 24, this means there should be 12 electron pairs Because there is one carbon-oxygen double bond and two carbon-oxygen single bond, two more structures need to be drawn with the double bond on a different oxygen
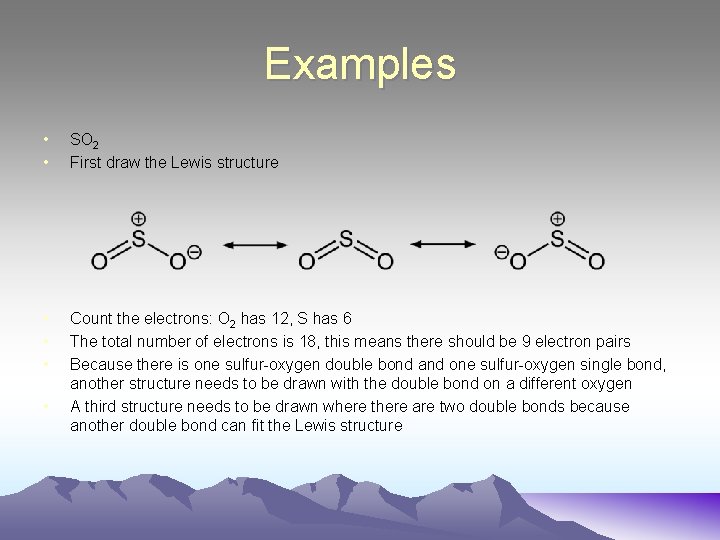
Examples • • SO 2 First draw the Lewis structure • • • Count the electrons: O 2 has 12, S has 6 The total number of electrons is 18, this means there should be 9 electron pairs Because there is one sulfur-oxygen double bond and one sulfur-oxygen single bond, another structure needs to be drawn with the double bond on a different oxygen A third structure needs to be drawn where there are two double bonds because another double bond can fit the Lewis structure •
- Slides: 6