Resonance Structures 1 Resonance Some molecules are have



- Slides: 7

Resonance Structures 1

Resonance • Some molecules are have structures that cannot be shown with a single representation • In these cases we draw structures that contribute to the final structure but which differ in the position of the bond(s) or lone pair(s) • Such a structure is delocalized and is represented by resonance forms • The resonance forms are connected by a double-headed arrow 2

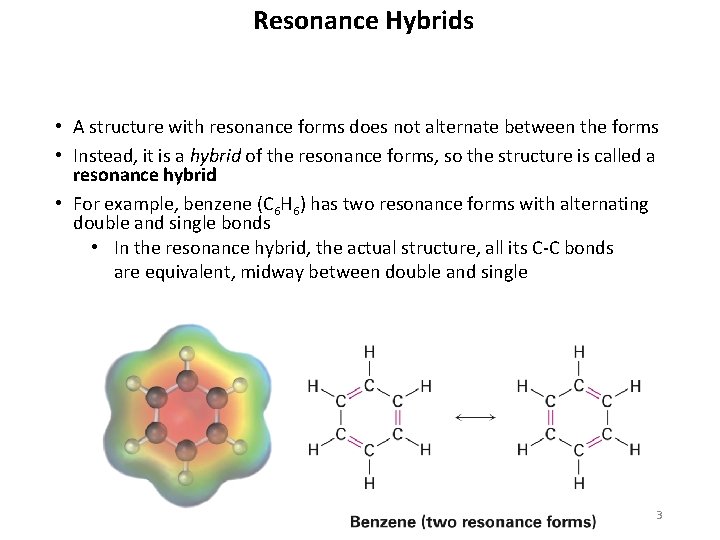
Resonance Hybrids • A structure with resonance forms does not alternate between the forms • Instead, it is a hybrid of the resonance forms, so the structure is called a resonance hybrid • For example, benzene (C 6 H 6) has two resonance forms with alternating double and single bonds • In the resonance hybrid, the actual structure, all its C-C bonds are equivalent, midway between double and single 3

Rules for Resonance Structures • You must have extra electrons, and somewhere to move them. • All structures must be valid Lewis Structures. • The true structure of a molecule is a hybrid or average of the individual resonance structures. It does not quickly shift back and forth between them. • Resonance structures increase the overall stability of the molecule compared with molecules lacking resonance structures. More structures = more stability. • Equivalent resonance structures all contribute equally to the resonance hybrid. • Nonequivalent resonance structures do not contribute equally; their relative stability determines how much they contribute. More stable structures contribute more; in other words, the molecule "looks more" like the more stable structure(s). • To evaluate the relative stability of resonance structures: • Structures in which all atoms (except hydrogen) have a complete octet are especially stable and make larger contributions to the hybrid. • Structures with fewer and lower formal charges contribute more. • If structures have the same number of atoms with formal charges, look at placement of charges. Structures more stable when negative charge on atom with higher EN, and when positive charge on less EN atom. 4

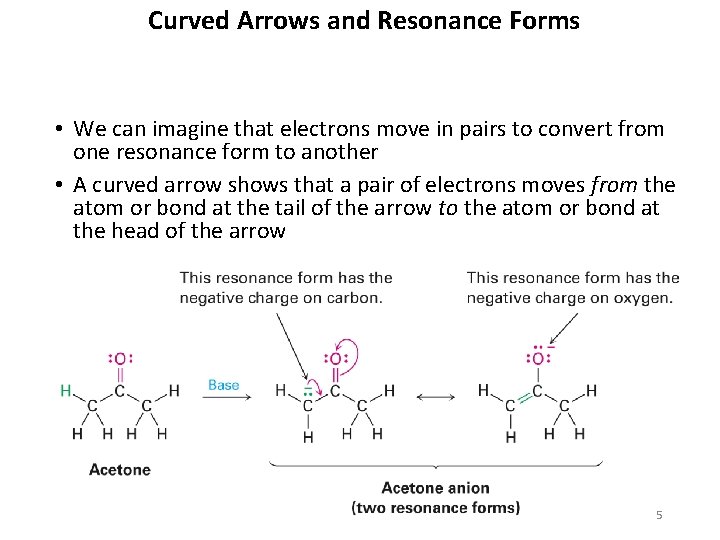
Curved Arrows and Resonance Forms • We can imagine that electrons move in pairs to convert from one resonance form to another • A curved arrow shows that a pair of electrons moves from the atom or bond at the tail of the arrow to the atom or bond at the head of the arrow 5

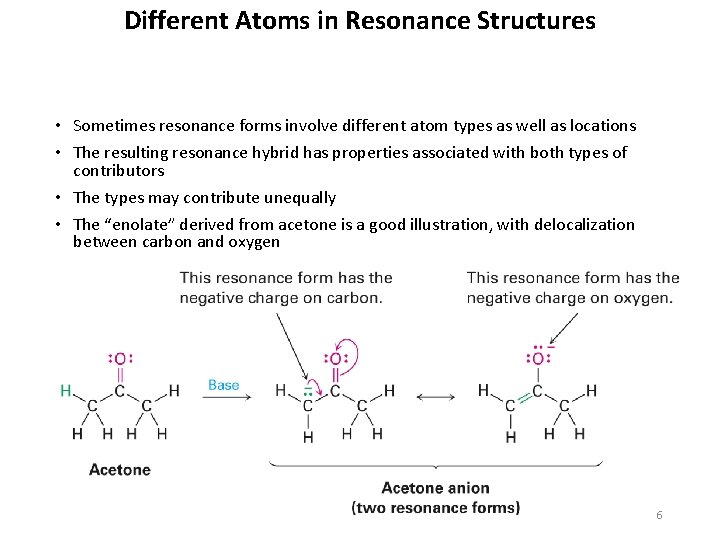
Different Atoms in Resonance Structures • Sometimes resonance forms involve different atom types as well as locations • The resulting resonance hybrid has properties associated with both types of contributors • The types may contribute unequally • The “enolate” derived from acetone is a good illustration, with delocalization between carbon and oxygen 6

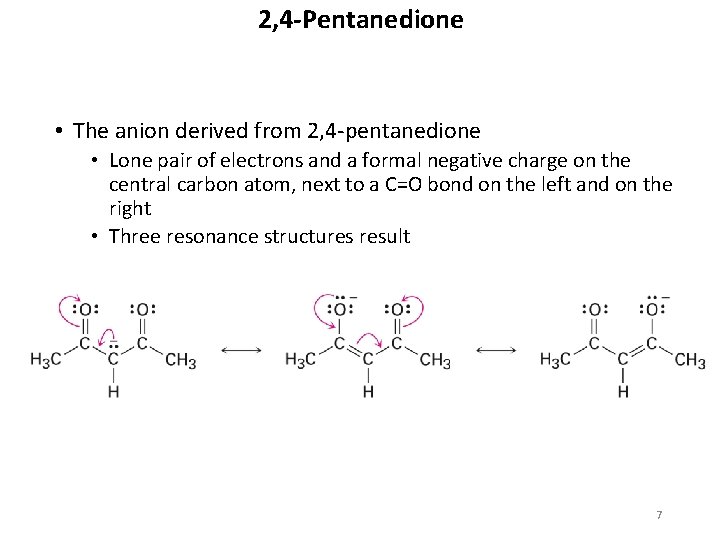
2, 4 -Pentanedione • The anion derived from 2, 4 -pentanedione • Lone pair of electrons and a formal negative charge on the central carbon atom, next to a C=O bond on the left and on the right • Three resonance structures result 7