Resonance and Formal Charge 1 Resonance and Formal







- Slides: 27

Resonance and Formal Charge 1

Resonance and Formal Charge: At the conclusion of our time together, you should be able to: 1. Define resonance 2. Determine resonance structures for a molecule 3. Calculate the formal charge for an atom 4. Determine the resonance structure that contributes the most to a compound by using formal charge 2

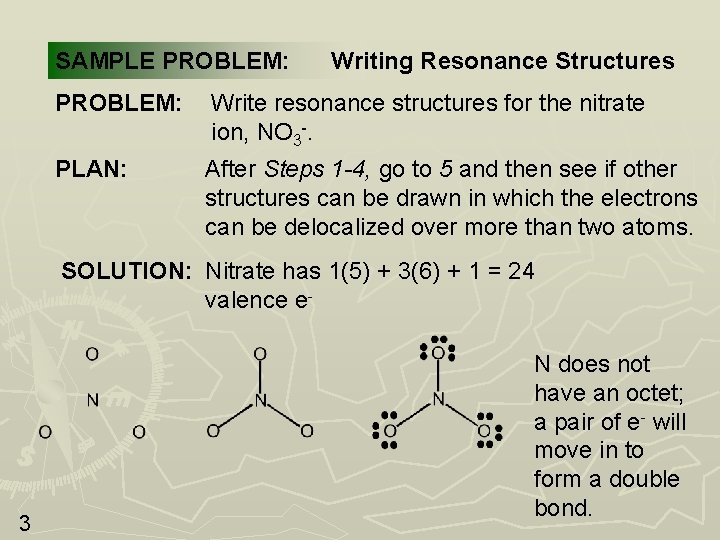
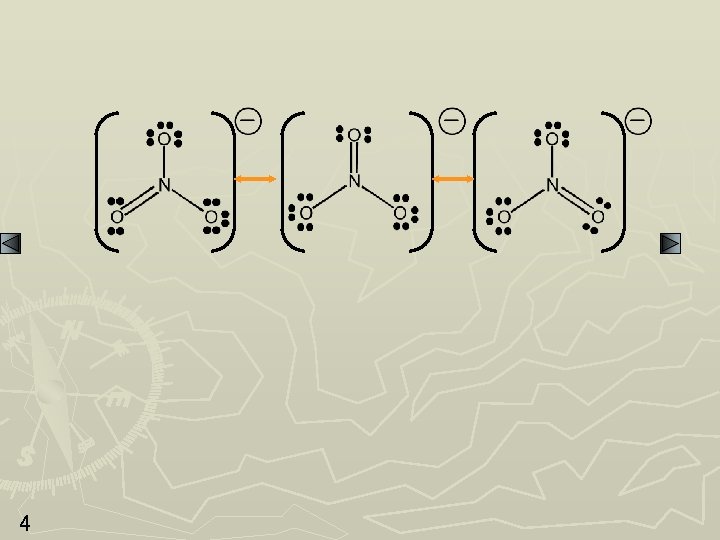
SAMPLE PROBLEM: Writing Resonance Structures PROBLEM: Write resonance structures for the nitrate ion, NO 3 -. PLAN: After Steps 1 -4, go to 5 and then see if other structures can be drawn in which the electrons can be delocalized over more than two atoms. SOLUTION: Nitrate has 1(5) + 3(6) + 1 = 24 valence e- 3 N does not have an octet; a pair of e- will move in to form a double bond.

4

Four criteria for choosing the more important resonance structure: 1. Smaller formal charges (either positive or negative) are preferable to larger charges; 2. A more negative formal charge should exist on an atom with a larger EN value. 3. Get unlike charges as close together as possible 5 4. Avoid like charges (+ + or - - ) on adjacent atoms

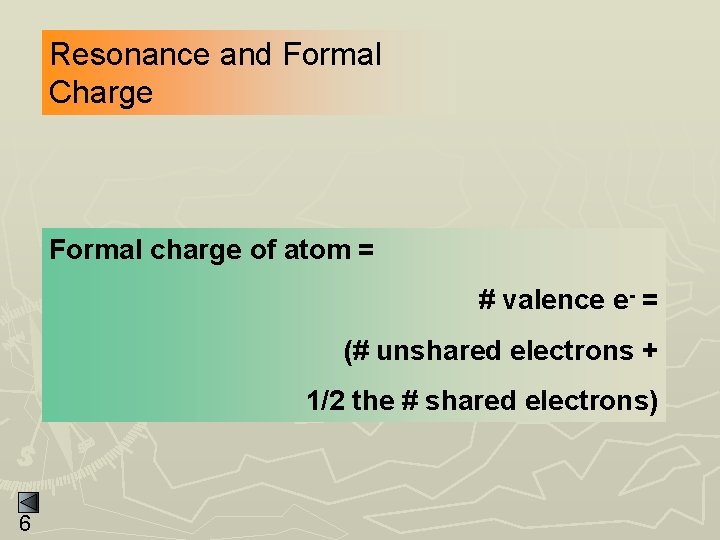
Resonance and Formal Charge Formal charge of atom = # valence e- = (# unshared electrons + 1/2 the # shared electrons) 6

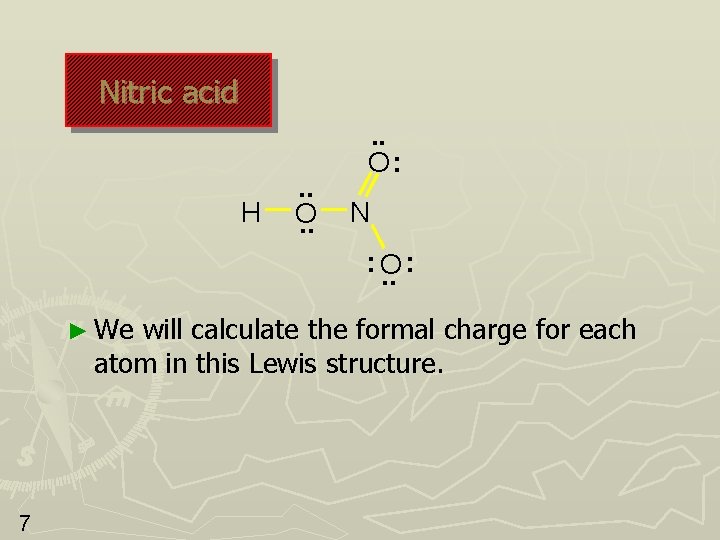
Nitric acid H . . O: N : O. . : ► We will calculate the formal charge for each atom in this Lewis structure. 7

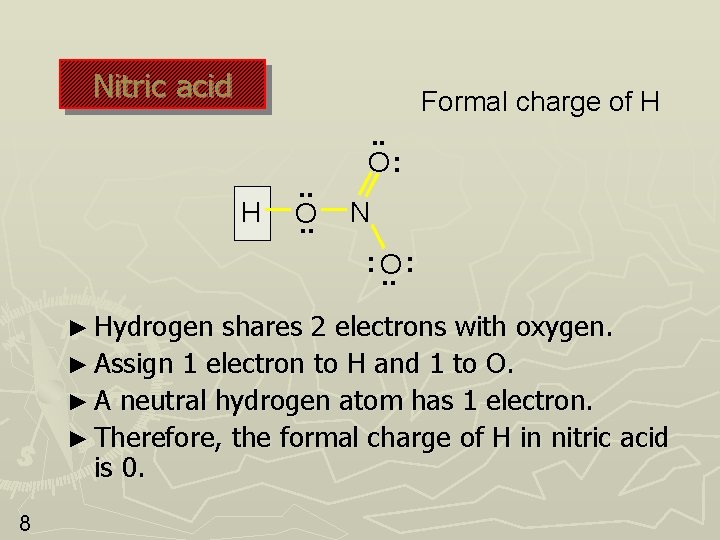
Nitric acid Formal charge of H H . . O: N : O. . : ► Hydrogen shares 2 electrons with oxygen. ► Assign 1 electron to H and 1 to O. ► A neutral hydrogen atom has 1 electron. ► Therefore, the formal charge of H in nitric acid is 0. 8

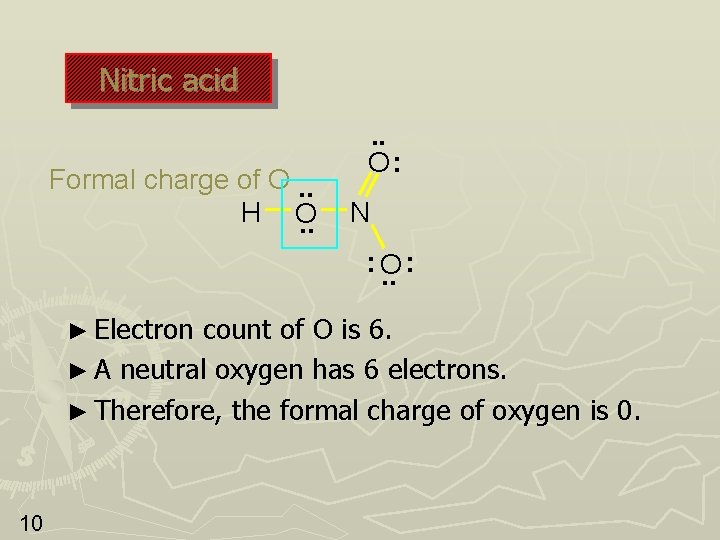
Nitric acid Formal charge of O. . H O. . O: N : O. . : ► Oxygen 9 has 4 electrons in covalent bonds. ► Assign 2 of these 4 electrons to O. ► Oxygen has 2 unshared pairs. Assign all 4 of these electrons to O. ► Therefore, the total number of electrons assigned to O is 2 + 4 = 6.

Nitric acid Formal charge of O. . H O. . O: N : O. . : ► Electron count of O is 6. ► A neutral oxygen has 6 electrons. ► Therefore, the formal charge of oxygen is 0. 10

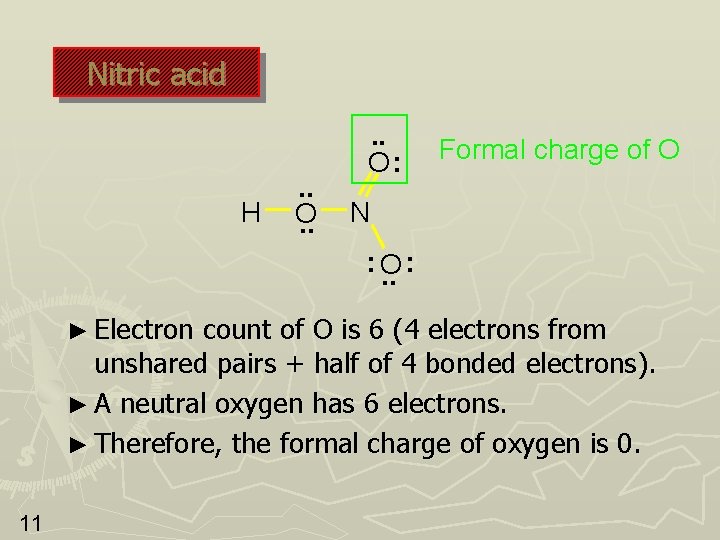
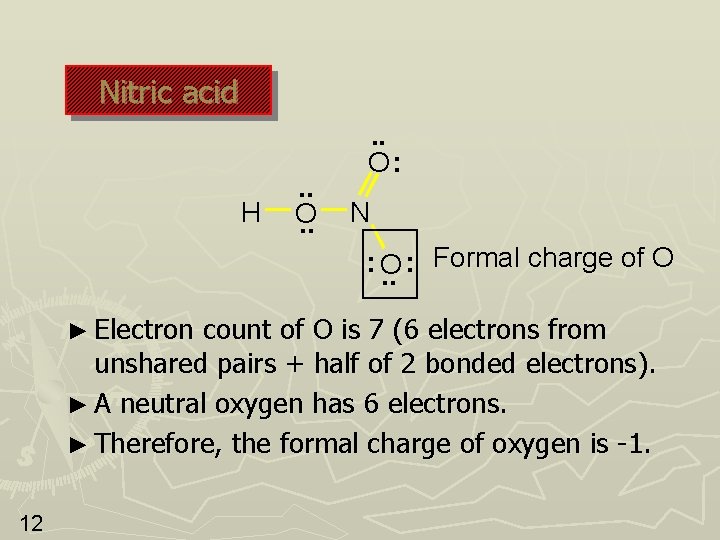
Nitric acid H . . O: Formal charge of O N : O. . : ► Electron count of O is 6 (4 electrons from unshared pairs + half of 4 bonded electrons). ► A neutral oxygen has 6 electrons. ► Therefore, the formal charge of oxygen is 0. 11

Nitric acid H . . O: N Formal charge of O : : O. . ► Electron count of O is 7 (6 electrons from unshared pairs + half of 2 bonded electrons). ► A neutral oxygen has 6 electrons. ► Therefore, the formal charge of oxygen is -1. 12

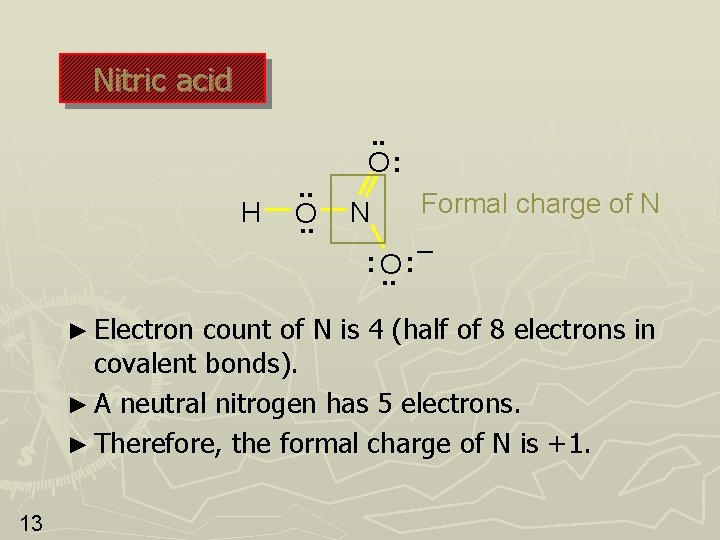
Nitric acid H . . O: N : O. . ► Electron Formal charge of N – : count of N is 4 (half of 8 electrons in covalent bonds). ► A neutral nitrogen has 5 electrons. ► Therefore, the formal charge of N is +1. 13

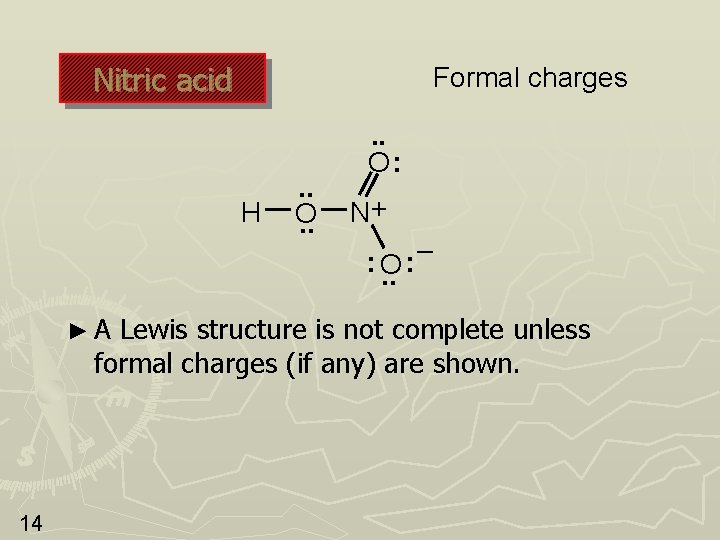
Nitric acid Formal charges H . . O: N+ : O. . ►A – : Lewis structure is not complete unless formal charges (if any) are shown. 14

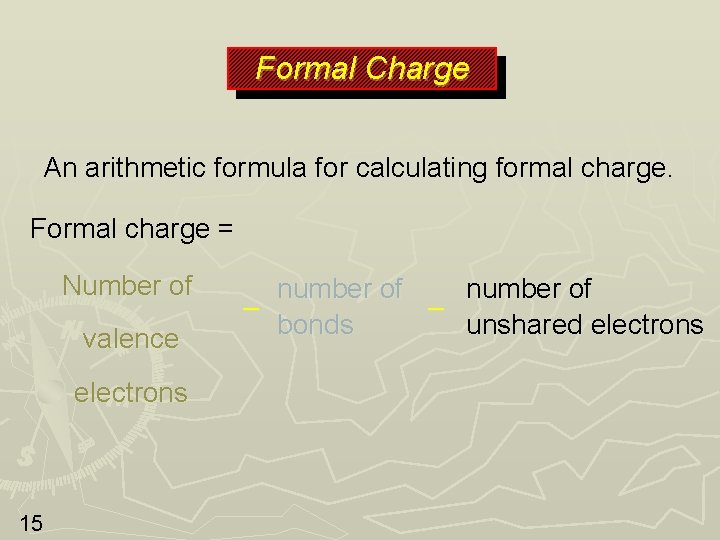
Formal Charge An arithmetic formula for calculating formal charge. Formal charge = Number of valence electrons 15 number of – – bonds unshared electrons

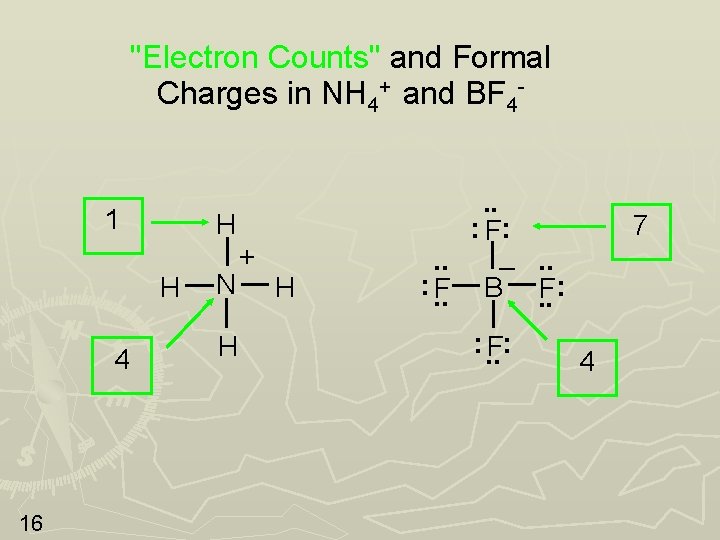
"Electron Counts" and Formal Charges in NH 4+ and BF 4 - 1 H H 4 16 N H + H . . : F: . . –. . : . . F B. . F: : . . F: 7 4

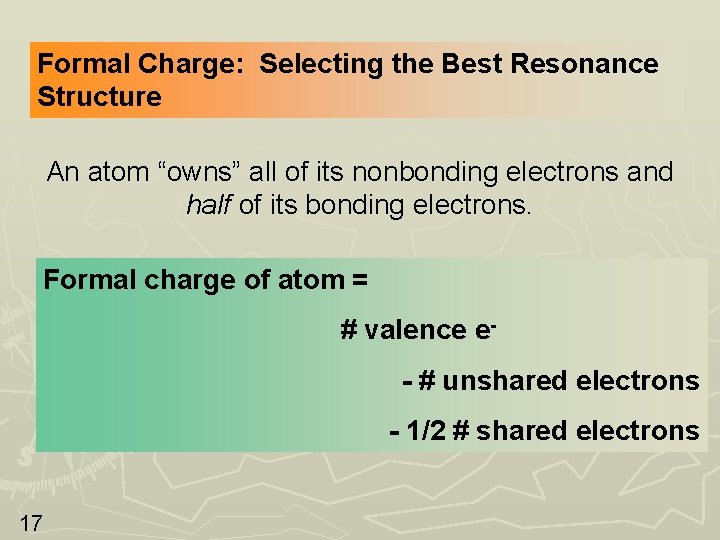
Formal Charge: Selecting the Best Resonance Structure An atom “owns” all of its nonbonding electrons and half of its bonding electrons. Formal charge of atom = # valence e- # unshared electrons - 1/2 # shared electrons 17

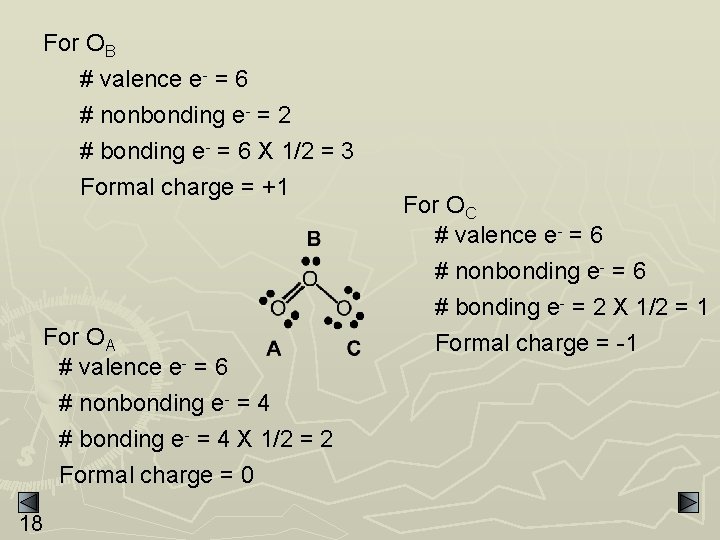
For OB # valence e- = 6 # nonbonding e- = 2 # bonding e- = 6 X 1/2 = 3 Formal charge = +1 For OA # valence e- = 6 # nonbonding e- = 4 # bonding e- = 4 X 1/2 = 2 Formal charge = 0 18 For OC # valence e- = 6 # nonbonding e- = 6 # bonding e- = 2 X 1/2 = 1 Formal charge = -1

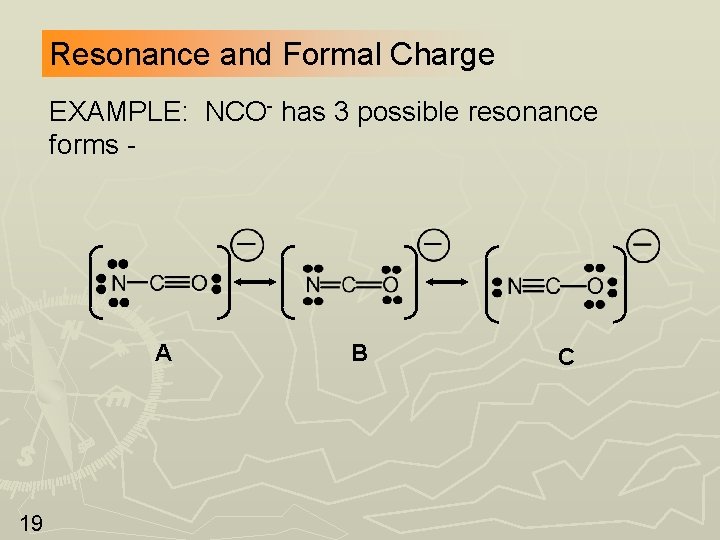
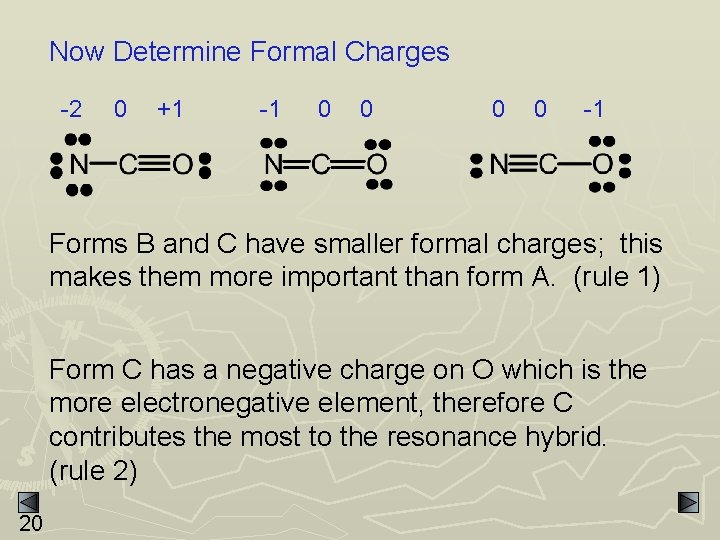
Resonance and Formal Charge EXAMPLE: NCO- has 3 possible resonance forms - A 19 B C

Now Determine Formal Charges -2 0 +1 -1 0 0 -1 Forms B and C have smaller formal charges; this makes them more important than form A. (rule 1) Form C has a negative charge on O which is the more electronegative element, therefore C contributes the most to the resonance hybrid. (rule 2) 20

Exceptions to the Octet Rule 21

Exceptions to the Octet Rule ► 4 b. Expanded Octets – only on period 3 and higher § Expanded octets form when an atom can decrease (or maintain at 0) it’s formal charge § Ex: SF 6, PCl 5, SO 2, SO 3, SO 4 ► 5 a. Electron deficient – have fewer than 8 § Ex: Be. Cl 2, BF 3 § may attain an octet by coordinate covalent bond ► Odd number of electrons – aka free radicals § Ex: NO 2 § May attain an octet by pairing with another free radical 22

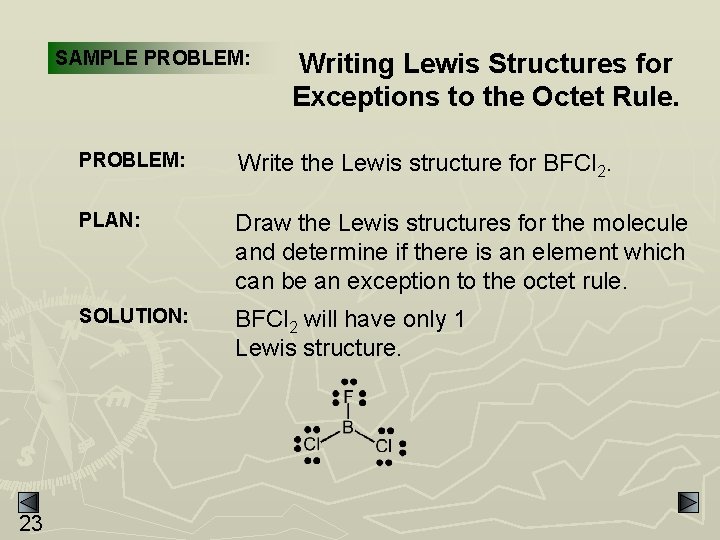
SAMPLE PROBLEM: 23 Writing Lewis Structures for Exceptions to the Octet Rule. PROBLEM: Write the Lewis structure for BFCl 2. PLAN: Draw the Lewis structures for the molecule and determine if there is an element which can be an exception to the octet rule. SOLUTION: BFCl 2 will have only 1 Lewis structure.

Resonance and Formal Charge: Let’s see if you can: 1. Define resonance 2. Determine resonance structures for a molecule 3. Calculate the formal charge for an atom 4. Determine the resonance structure that contributes the most to a compound by using formal charge 24

Your Turn Now determine the formal charges and best structure for the 2 examples at the bottom of page 11. 25

Your Turn Now determine the formal charges and best structure for the middle example on page 12. 26

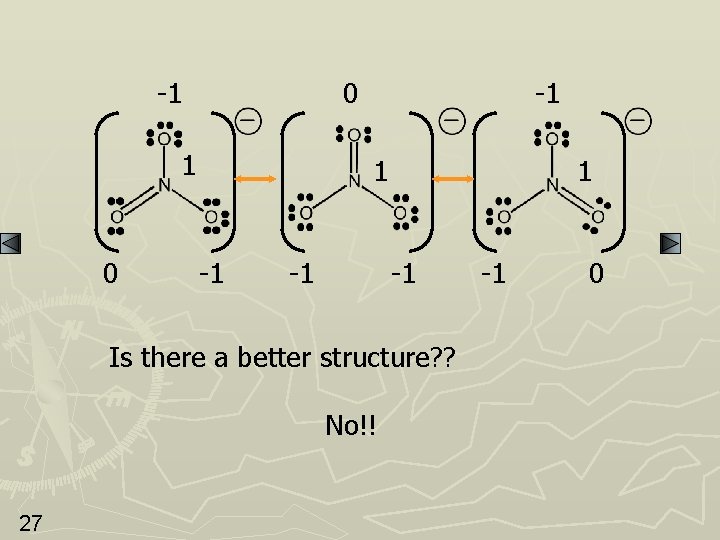
-1 0 -1 1 -1 Is there a better structure? ? No!! 27 -1 0