Residue Monitoring System Issuance of Health Certificate GAP




























- Slides: 52

Residue Monitoring System Issuance of Health Certificate GAP including ethical practices in export MAMTA RANI ASSISTANT DIRECTOR 06 September 2011 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 1

About Us • Export (QC&I) Act, 1963 – The Act governing quality of exports • EIC set up to advise Govt. on measures for sound development of exports through QC & I to include notification of standards & certification systems • Powers of Central Government under the Act Ø Notify commodities for compulsory PSI Ø Specify standards for export and type of QC & I Ø Establish or recognize Agencies for QC & I • Nearly 1000 commodities were notified. 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 2

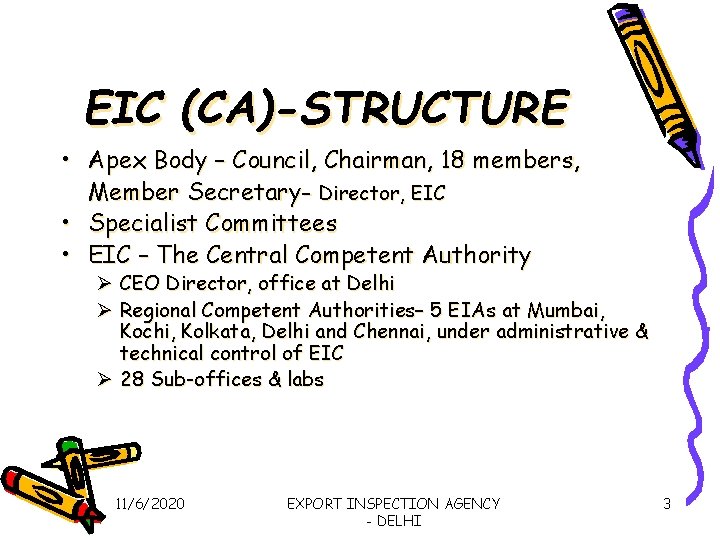
EIC (CA)-STRUCTURE • Apex Body – Council, Chairman, 18 members, Member Secretary- Director, EIC • Specialist Committees • EIC – The Central Competent Authority Ø CEO Director, office at Delhi Ø Regional Competent Authorities– 5 EIAs at Mumbai, Kochi, Kolkata, Delhi and Chennai, under administrative & technical control of EIC Ø 28 Sub-offices & labs 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 3

SYSTEMS OF EXPORT INSPECTION & CERTIFICATION • • • Consignment wise inspection In-Process Quality Control Self-Certification • Approval and monitoring of processing and manufacturing units based on food safety management systems such as GMP/ GHP / HACCP. 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 4

EIC-ROLE IN WTO REGIME • • • Regulatory role to Ø address health & safety concerns of importing countries Ø compulsory certification for Marine products, Egg products, Milk products, Honey products, Poultry Meat products etc. Voluntary export certification – Tea, F&V, Spices, Basmati Rice Equivalence Agreements/MOUs with trading partners for recognition of EIC’s certification Certificate of Health (Food items), Authenticity (Basmati Rice. EC) Laboratory Testing Ø Support for Export Inspection & Certification Ø Commercial testing (facilities extended to industry) Ø Import testing of food items-EIA Labs identified by Mo. H&FW 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 5

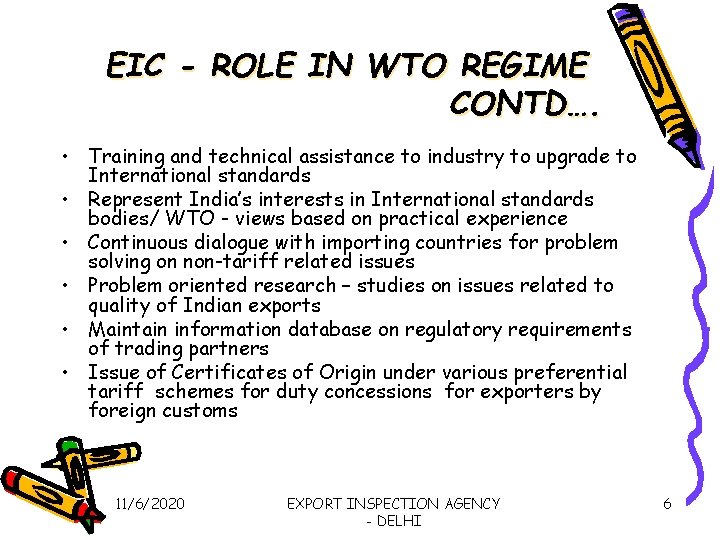
EIC - ROLE IN WTO REGIME CONTD…. • Training and technical assistance to industry to upgrade to International standards • Represent India’s interests in International standards bodies/ WTO - views based on practical experience • Continuous dialogue with importing countries for problem solving on non-tariff related issues • Problem oriented research – studies on issues related to quality of Indian exports • Maintain information database on regulatory requirements of trading partners • Issue of Certificates of Origin under various preferential tariff schemes for duty concessions for exporters by foreign customs 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 6

INTERNATIONAL RECOGNITIONS • • • EC - CA for marine products and issuance of authenticity certificate for basmati rice; egg products, dialogue on for dairy products, poultry meat & honey Italy- MOU for marine products. USA (USFDA) - recognized for Black Pepper; initiated dialogue for others. Australia (AQIS) - recognized for marine products –seeking for dairy products, spices etc. Sri Lanka (SLSI) - recognized for 85 products regulated by Sri Lanka (food, cement, engineering items, electrical appliances, milk products etc. Singapore – The MRA’s covers Food & Agriculture. Korea- Recognized for certifying food products Japan- Recognized for certifying F& FP, Poultry products Russia – Recognized for Marine Products Others - Other EU countries, Canada, Argentina etc- under negotiation 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 7

Our website (www. eicindia. org) 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 8

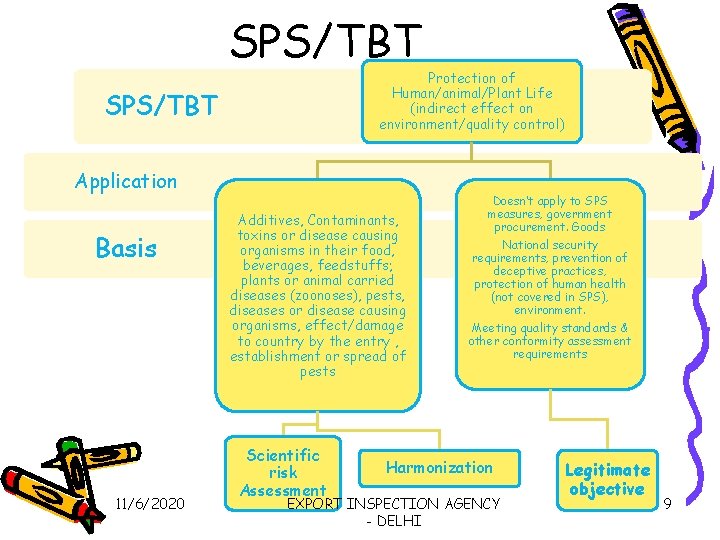
SPS/TBT Protection of Human/animal/Plant Life (indirect effect on environment/quality control) SPS/TBT Application Basis 11/6/2020 Additives, Contaminants, toxins or disease causing organisms in their food, beverages, feedstuffs; plants or animal carried diseases (zoonoses), pests, diseases or disease causing organisms, effect/damage to country by the entry , establishment or spread of pests Scientific risk Assessment Doesn’t apply to SPS measures, government procurement. Goods National security requirements, prevention of deceptive practices, protection of human health (not covered in SPS), environment. Meeting quality standards & other conformity assessment requirements Harmonization EXPORT INSPECTION AGENCY - DELHI Legitimate objective 9

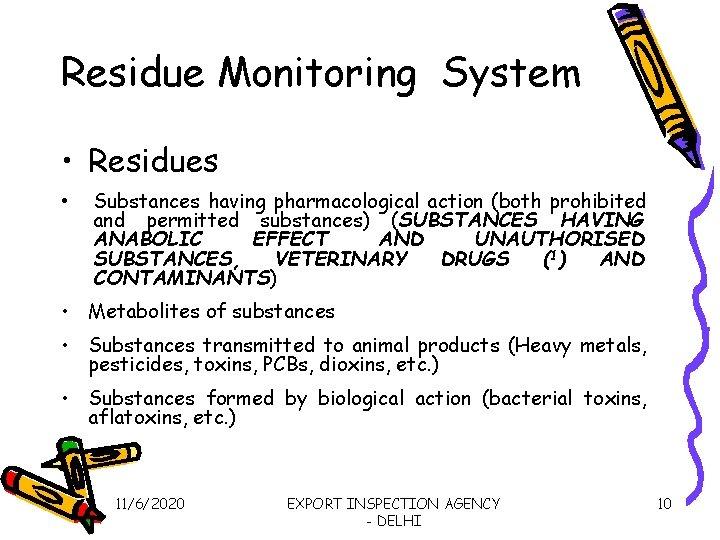
Residue Monitoring System • Residues • Substances having pharmacological action (both prohibited and permitted substances) (SUBSTANCES HAVING ANABOLIC EFFECT AND UNAUTHORISED SUBSTANCES, VETERINARY DRUGS (1) AND CONTAMINANTS) • Metabolites of substances • Substances transmitted to animal products (Heavy metals, pesticides, toxins, PCBs, dioxins, etc. ) • Substances formed by biological action (bacterial toxins, aflatoxins, etc. ) 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 10

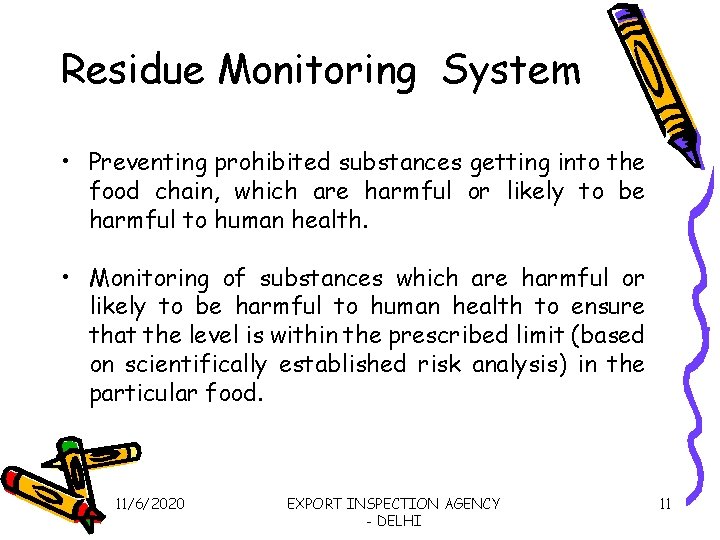
Residue Monitoring System • Preventing prohibited substances getting into the food chain, which are harmful or likely to be harmful to human health. • Monitoring of substances which are harmful or likely to be harmful to human health to ensure that the level is within the prescribed limit (based on scientifically established risk analysis) in the particular food. 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 11

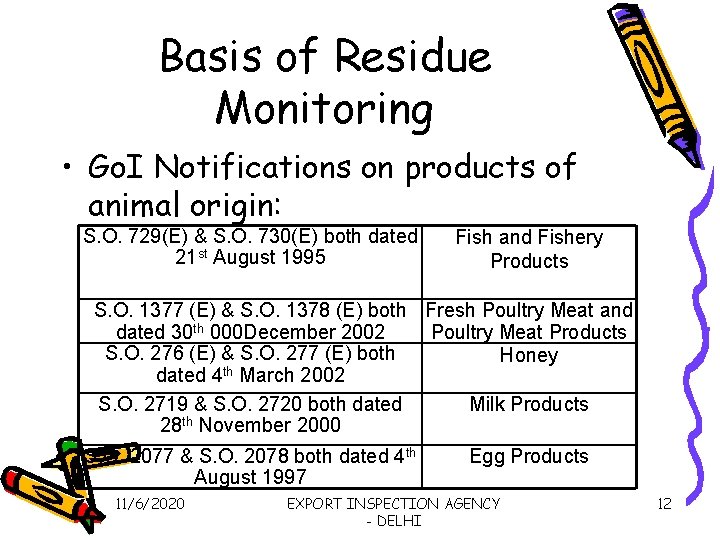
Basis of Residue Monitoring • Go. I Notifications on products of animal origin: S. O. 729(E) & S. O. 730(E) both dated 21 st August 1995 Fish and Fishery Products S. O. 1377 (E) & S. O. 1378 (E) both Fresh Poultry Meat and dated 30 th 000 December 2002 Poultry Meat Products S. O. 276 (E) & S. O. 277 (E) both Honey th dated 4 March 2002 S. O. 2719 & S. O. 2720 both dated Milk Products 28 th November 2000 S. O. 2077 & S. O. 2078 both dated 4 th August 1997 11/6/2020 Egg Products EXPORT INSPECTION AGENCY - DELHI 12

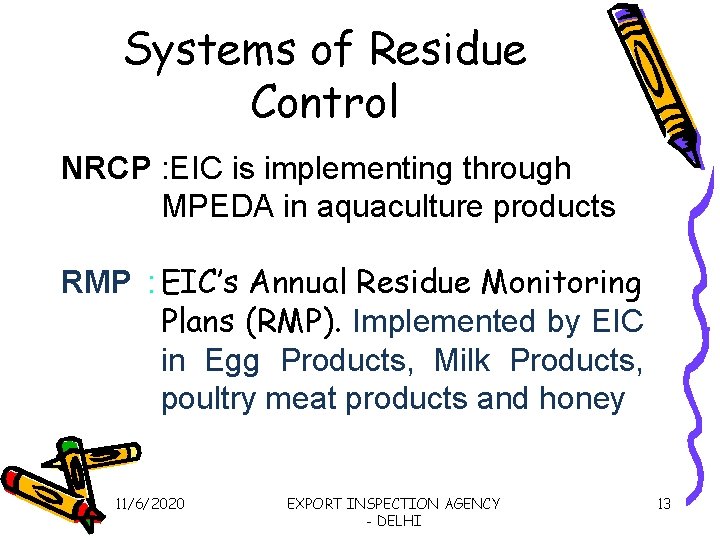
Systems of Residue Control NRCP : EIC is implementing through MPEDA in aquaculture products RMP : EIC’s Annual Residue Monitoring Plans (RMP). Implemented by EIC in Egg Products, Milk Products, poultry meat products and honey 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 13

Why NRCP and RMP? • • WTO Agreement: 1. SPS Agreement: Compliance of Sanitary and Phyto-Sanitary requirements of importing country- setting up country specific standards based on risk assessment & scientific evidences, Harmonization, Transparency, Mutual recognition 2. TBT Agreement: No importing country should impose Technical Barrier to Trade. Global food trade is Food Safety (The food free from health hazards). Consumer is entitled for safe, sound and wholesome food free from any physical, chemical and microbiological hazards Requirement of Harmonization SPS and TBT agreements require National Standards to Harmonize with International ones 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 14

NRCP and RMP • Objective: § Monitoring and insurance of the acceptable residue levels of drugs, pesticides and contaminants in the food products. § Detection of any illegal treatment (s) § Establishing a system of corrective action in the event of detection of residues higher that the prescribed limits by issuing alert information and follow up visits § To ensure that the food products exported from India meet the prescribed regulatory requirements of the importing countries. • Scope: • Animals or animal origin products and processing meant for export by the approved processing establishments having implemented HACCP based own check system. 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 15

NRCP/RMP for EU • To fulfill the requirements of EU – EC is responsible to ensure high level of human health protection through its Food & Feed regulations. – EU food safety policy as 178/2002/EC for food and feed with integrated "farm to fork" approach, aims to harmonise existing national requirements in order to ensure the free movement of food and feed in the EU. – For import from third countries, - EC seeks guarantees equivalent to EC requirements on residues of veterinary drugs, pesticides and contaminants 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 16

Basis of animal origin products ü Council Directive 96/23/EC dated 29. 04. 1996, stipulated in the Council Directive 96/22/EC as amended by Council Directive 2003/74/EC – requirements in relation to the planning and execution of national residue control plans (NRCP) for live animals and products of animal origin (third countries) ü Commission Regulation (EC) No. 136/2004 – authorizes CAs of member states of EU to test samples for residues at BIPs (Border Inspection Posts) ü Directive 97/78/EC - EU or the individual member state may reinforce checks at the point of import on identification of residue problem 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 17

NRCP/RMP for EU ü Council Directive 86/363/EEC and Commission Regulation (EC) 1881/2006 - Maximum Residue Levels (MRLs) for a wide range of pesticides and maximum levels (MLs) for certain environmental contaminants, respectively. ü Annual submission – RMP for each food commodity from third countries to EC, (plans + previous year results by the 31 st March every year) ü Approval of Plan by EC- listed in the Commission Decision 2004/432/EC, (updated). ü According to the last update India is eligible for export the products of aquaculture, eggs to EU. 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 18

Basis for plant products • In the EU, as from 1 September 2008, a new legislative framework (Regulation (EC) No 396/2005 on pesticide residues is applicable, • Covering approx. 1100 pesticides currently or formerly used in agriculture in or outside the EU. • It lists MRLs for 315 agricultural products. These MRLs also apply to processed products, adjusted to take account of dilution or concentration during processing. • To access the database, http: //ec. europa. eu/sanco_pesticides/public/index. cf m • EFSA - responsible for the safety assessment. 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 19

Benefits of RMP implementation Food products of animal origin • • Supplier / farmer - food safety measures - safe food products Establishment - controls / monitoring over farmer / supplier at primary production, storage and transportation levels. All the stakeholders in the food chain - safe food products for human consumption FP meant for export - free from prohibited substances. Permitted level of Veterinary drugs / pharmacologically active substances, Pesticide residues, Heavy metals, etc. would be monitored. Increase in Demand of safe food products – Increase in Export earning Farmers - benefited from safe production - employment and earnings 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 20

NRCP – Salient features Implemented by MPEDA • EIC - CCA • Sampling from aquaculture farms and testing, as per schedule - by MPEDA • Test results compiled and submitted to EIC. • In case of failure of sample, if any, EIA concerned will initiate appropriate action to find root cause and prevent recurrence. Also, furnishes action taken report to EIC 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 21

RMP – Salient features Implemented by EIC - EIC – CCA, EIA- RCA Responsibilities of processors§ To ensure that the registered feed mills supplying feed to the producers / farmers and farms / producers supplying animal products) – records – EIA M. O Sampling Procedure: • • Multiple sampling of same matrix from same source - avoided The Representative samples - traceable to source of farm / producer Samples - collected and secured in clean and inert containers & labelled Samples should be sent to designated EIA lab/ EIC approved lab under controlled conditions. 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 22

RMP – Salient features Analysis and handling of samples: • The sample - secured storage at appropriate storage condition. • Analysis - as per the protocol given in the RMP by EIC. • Remaining samples - stored securely at appropriate condition. • Initial test - positive, the remaining sample shall be analysed for confirmation of results. • The test results shall be reported in the prescribed format • In case of failure, the results communicated immediately to the EIA concerned 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 23

RMP – Salient features Corrective Action in case of failure of samples: Processor to be kept on ‘ On-Alert’ and advisory issued to exporters, for; Ø Identification of the exact source Ø Stop procurement of raw material from the source. Ø Refrain from exporting the products processed from the identified source Ø Find the root cause for the failure of the samples from the identified source Ø Take corrective actions to prevent recurrence Ø Review the HACCP and revise if necessary Ø Conduct regular training for farmers / producers / suppliers to prevent recurrence 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 24

RMP – Salient features Corrective Action in case of failure of samples: (Contd. ) Ø Assessment by the EIA official to find out the source and root cause of the contamination including backward linkages and assists in identifying preventative measure to stop the recurrence Ø The live stock concerned and the product is kept under official control Ø The source producer/farm is subjected to more checks 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 25

RMP – Salient features • Provision for re-testing of positive samples § § Re-testing of the positive samples - on request from the unit ( reconfirmation). The control sample - tested in two different EIA labs/ EIC approved labs other than one tested earlier. The result to be treated as positive even if one of the two samples is found to be positive on re-testing. In case both the samples pass the MRL requirement on re-testing, the concerned EIA shall withdraw internal alert , which shall take effect from that date. –Recording and reporting: § § All records relevant to planning, sampling and testing to be maintained at EIAs. monthly / quarterly summary in the prescribed format to be submitted by the Sub-office to the EIA concerned, in the end to EIC 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 26

Issuance of Health Certificates • What is HC? – A document for a consignment of food product certifying that: – the consignment has been processed under proper sanitary and hygienic conditions, and that – the food product is safe for human consumption. issued by a competent authority, acceptable to the health authority of the importing country. 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 27

Health Certificates • Who demands? – Importing countries insist on Health / Veterinary / Sanitary/ Phyto-sanitary. • Why ? - provides confidence to the health authorities of the importing country 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 28

Health Certificates • Issuing authority? : Competent authority specified by the importing country. – Export Inspection Council of India – CA for F&FP meant for export to EU, countries other than EU. – EIA issues HC on behalf of EIC using the official rubber stamp (seal) of EIC – Some countries accept HC issued by official veterinary authorities. – If an EU approved establishment obtains HC from any – body other than EIC/EIA, its approval is liable to be withdrawn 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 29

Requisites to issue HC • On request from approved processor/exporter • On the basis of controls carried out, - on or before the date of shipment • HC not issued after the date of shipment (indicated in the Bill of Lading) Note: HC is issued only for F&FP processed in establishments/factory vessels/freezer vessels approved by EIC/EIA, likewise milk products, egg products, honey etc. 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 30

Requisites to issue HC • HC for consignments of F&FP meant for non-EU countries to be issued by the EIA concerned in the prescribed format given at Annexure XXII(A) of EI • If any country has prescribed format of HC, the specific format will be used as required by the importing country. For e. g. Australia 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 31

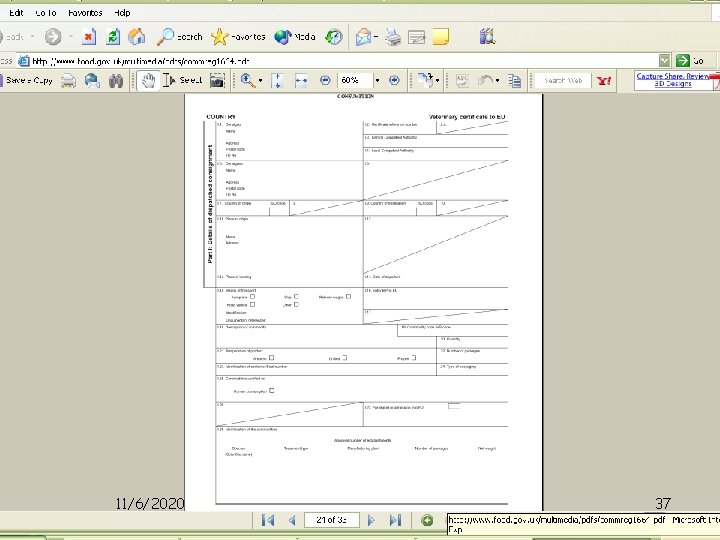
Formats of HC • COMMISSION REGULATION (EC) No 1664/2006 – for HONEY AND OTHER APICULTURE PRODUCTS • COMMISSION REGULATION (EC) No 1250/2008 – • FISHERY PRODUCTS INTENDED FOR HUMAN • CONSUMPTION • COMMISSION REGULATION (EU) No 364/2011 - for egg products (EP) HC is prepared in triplicate: (Blue) for office record 11/6/2020 Original (White) for importer; Duplicate (Pink) for HO; Triplicate EXPORT INSPECTION AGENCY - DELHI 32

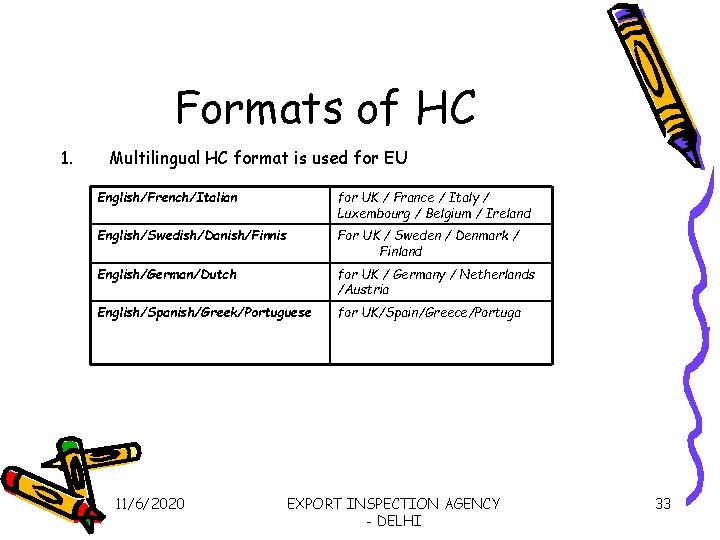
Formats of HC 1. Multilingual HC format is used for EU English/French/Italian for UK / France / Italy / Luxembourg / Belgium / Ireland English/Swedish/Danish/Finnis For UK / Sweden / Denmark / Finland English/German/Dutch for UK / Germany / Netherlands /Austria English/Spanish/Greek/Portuguese for UK/Spain/Greece/Portuga 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 33

11/6/2020 EXPORT INSPECTION AGENCY - DELHI 34

11/6/2020 EXPORT INSPECTION AGENCY - DELHI 35

11/6/2020 EXPORT INSPECTION AGENCY - DELHI 36

11/6/2020 EXPORT INSPECTION AGENCY - DELHI 37

Certificate of Analysis for Aquaculture Shrimps meant for export to Japan • If processor/exporter approaches EIA - consignments of Aquaculture Shrimps meant for export to Japan • for the samples shall be drawn by an authorized EIA officer and the same shall be tested for Antibiotic Residues including Nitrofuran Metabolites, in EIA lab under its jurisdiction. • The cost of testing to be borne by the processor. • Format of Certificate of Analysis to be issued is given at Annexure XXVIII of Ex. Instructions. 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 38

Best Agriculture Practices in export • Reforming agriculture and making the produce internationally competitive in quality & food safety. • Green revolution – uncontrolled usage of chemical fertilizers, irrigation water & pest control products – adverse environment impact, degradation & increased salinity in soil, deforestation and depletion of water resources. 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 39

GAP- role in export • Structured methodology, innovative technology without its adverse impact on environment, health & safety of people. • GAP – collection of principles – on farm production, post production processes – • - integrated pest management • - integrated fertilizer management & • - conservation agriculture 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 40

Basic elements of GAP • • • Farm selection & Farm management Rational use & application of pesticides Judicious use of fertilizers Soil conservation & soil management Irrigation & water conservation Integrated pest management Produce storage & handling Pre – harvest application of pesticides Harvesting practices 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 41

Basic elements of GAP Contd…. • Post harvest treatment • Workers health, safety & welfare • Traceability – from farm to fork/back tracking from fork to farm, batch/lot recall • Protection of environment 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 42

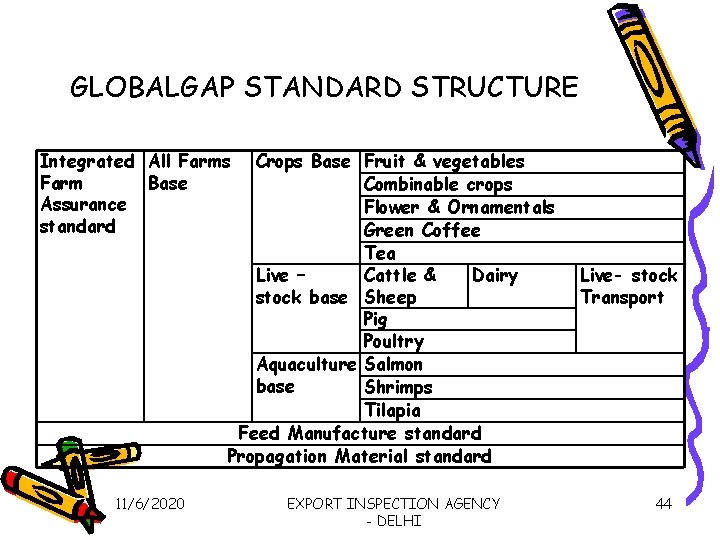
Standardization of good agricultural Practices • Global. GAP - key reference for GAP in global market place- GLOBALGAP Secretariat • Global. GAP standards cover entire range of agriculture including animal husbandry & fisheries – (next slide) • Consists of set of normative documents covering GLOBALGAP general regulations, the GLOBALGAP control points & Compliance criteria and the GLOBALGAP checklist. 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 43

GLOBALGAP STANDARD STRUCTURE Integrated All Farms Farm Base Assurance standard Crops Base Fruit & vegetables Combinable crops Flower & Ornamentals Green Coffee Tea Live – Cattle & Dairy stock base Sheep Pig Poultry Aquaculture Salmon base Shrimps Tilapia Feed Manufacture standard Propagation Material standard 11/6/2020 EXPORT INSPECTION AGENCY - DELHI Live- stock Transport 44

Harmonization with GLOBALGAP • Variations In Agriculture Practices – Country/Region Wise • Agro climatic variations, Cultural practices, regulatory framework • Two routes: • Introducing national requirements in GLOBALGAP Standards and getting its approval by Global GAP secretariat through National Technical working Group • Benchmarking national standards such as India. GAP, Malaysia. GAP, Kenya. GAP etc. - address essential elements good agriculture practices covered under Global. GAP 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 45

Harmonization with GLOBALGAP • In India, both the streams of standards – under the aegis of NTWG set up by QCI and another FSSAI with assistance from QCI • India GAP standards prepared - GAP-Basic requirements, fresh fruits & vegetables, combinable crops, green coffee, & tea 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 46

Certification of Good Agricultural Practices • More than 100 independent and accredited certification bodies in more than 80 countries. • Open to all producers worldwide • FSSAI led India. GAP cover: Ø Certification Criteria Ø Certification Process Ø Certification body requirements ‘Draft available at QCI website’ 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 47

Certification of Good Agricultural Practices • NABCB (National Accreditation Board for Certification Bodies) of QCI – • Accreditation of certification bodies based on ISO Guide 65 along with relevant scheme – Global. GAP or India. GAP • Two options: Ø Individual certification: Multisite without implementation of QMS, Multisite with implementation of QMS, Basic progressive model Ø Group certification 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 48

Certification of Good Agricultural Practices • Ensure conformity to standards on India. GAP standards Ø India. GAP-Standard intending for Bench marking with Global. GAP Ø India. GAP – basic requirements (progressive in nature) • Evaluation against control points and compliance criteria (CPCC) • Compliance to relevant statutory and regulatory requirement applicable in area of operation • Before certification process applicant shall: o o o Selection of farms for GAP certification Assigning identification number to the different units of the farm Establish legal identity & registration Selection of certification model depending upon nature of operation Training on India. GAP to farmers 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 49

Certification of Good Agricultural Practices • • • o Establishing documented system to meet the requirements & its implementation o Training of inspectors/auditors o Conducting internal evaluation and appraisal of the system by trained farm inspectors o Taking corrective action and improving the situation o Appointment of certification body Documents for introducing India. GAP certification: IGAP-01 – certification criteria (voluntary) IGAP-02 – Certification process (voluntary) IGAP-03 - group certification (voluntary) IGAP -04 – basic module (voluntary) IGAP -05 – certification body requirements (voluntary) 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 50

GAP Certification Process Applicant → Application ↓ Scrutiny Application ↓ Review of application ↓ Farm Inspection Audit Report ↓ Decision on certification ↓ Award of certificate → surveillance → Renewal 11/6/2020 EXPORT INSPECTION AGENCY - DELHI 51

11/6/2020 EXPORT INSPECTION AGENCY - DELHI 52
 Mode of issuance
Mode of issuance Snowtam format example
Snowtam format example Gasb 65 debt issuance costs
Gasb 65 debt issuance costs Complete residue system modulo 5
Complete residue system modulo 5 Mood adjunct examples
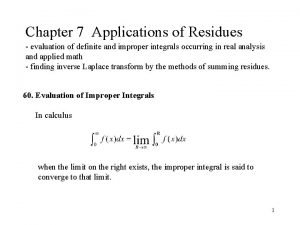
Mood adjunct examples Application of residue theorem to evaluate real integrals
Application of residue theorem to evaluate real integrals Simple pole
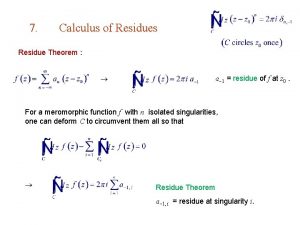
Simple pole Residue theorem
Residue theorem Interpersonal function examples
Interpersonal function examples Moral residue
Moral residue Residue theorem
Residue theorem European pesticide residue workshop
European pesticide residue workshop Ergashgan qoshma gapga misollar
Ergashgan qoshma gapga misollar Compiler bridges the semantic gap between which domains?
Compiler bridges the semantic gap between which domains? Care certificate standard 3 duty of care answers
Care certificate standard 3 duty of care answers Public health entomology certificate
Public health entomology certificate Public health entomology certificate
Public health entomology certificate Www.eicindia.gov.in health certificate
Www.eicindia.gov.in health certificate Usda health certificate
Usda health certificate Community health planning and implementation certificate
Community health planning and implementation certificate Structural health monitoring
Structural health monitoring Shm
Shm Comparative vacuum monitoring
Comparative vacuum monitoring Drinking water system operator certificate
Drinking water system operator certificate Language processor
Language processor Fuel monitoring system for vehicles
Fuel monitoring system for vehicles Poll monitoring system app
Poll monitoring system app Sms poll voting
Sms poll voting Sms based poll monitoring system
Sms based poll monitoring system Sms based patient monitoring system
Sms based patient monitoring system Structure of a project monitoring information system
Structure of a project monitoring information system Monitors
Monitors Azure geneva monitoring
Azure geneva monitoring Distance monitoring system
Distance monitoring system Reservoir water monitoring systems
Reservoir water monitoring systems Cho hwc performance monitoring system
Cho hwc performance monitoring system Food contamination monitoring system
Food contamination monitoring system Early warning intervention and monitoring system
Early warning intervention and monitoring system Centralized public grievance redress & monitoring system
Centralized public grievance redress & monitoring system Cathodic protection monitoring system
Cathodic protection monitoring system Swasthya sewa dapoon
Swasthya sewa dapoon Ece 445
Ece 445 Engine oil debris sensors
Engine oil debris sensors Ideal clinic monitoring
Ideal clinic monitoring Wigos data quality monitoring system
Wigos data quality monitoring system Mrunalini sawant
Mrunalini sawant Reservoir water level monitoring system
Reservoir water level monitoring system Network monitoring definitions
Network monitoring definitions Facility monitoring system
Facility monitoring system Nj sams
Nj sams Poll monitoring system
Poll monitoring system Single parameter monitoring system
Single parameter monitoring system Progress monitoring system
Progress monitoring system