Research Methodology Study Design 1 Davood Khalili MD










- Slides: 20


Research Methodology Study Design (1) Davood Khalili, MD, MPH, Ph. D Department of Biostatistics & Epidemiology Research Institute for Endocrine Sciences Shahid Beheshti University of Medical Sciences


Evidence to action needs research Conducting a research needs some tools Study designs are these tools

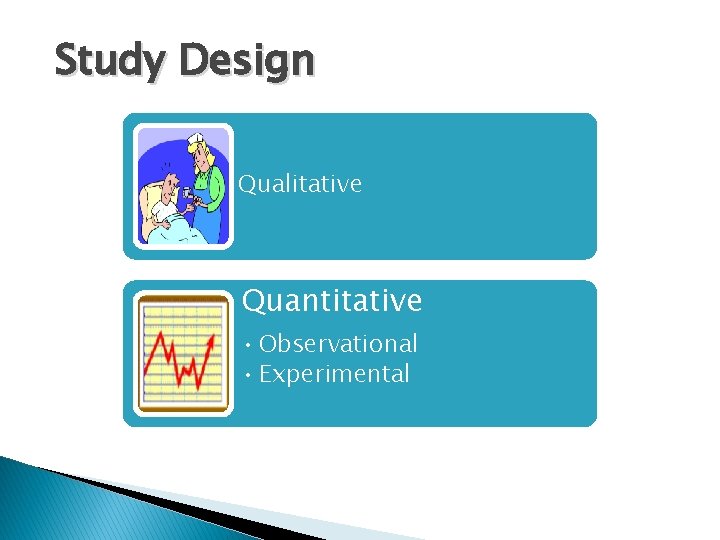
Study Design Categories Qualitative Quantitative Basic Applied Observational Experimental Descriptive Analytic

Study Design Qualitative Quantitative • Observational • Experimental

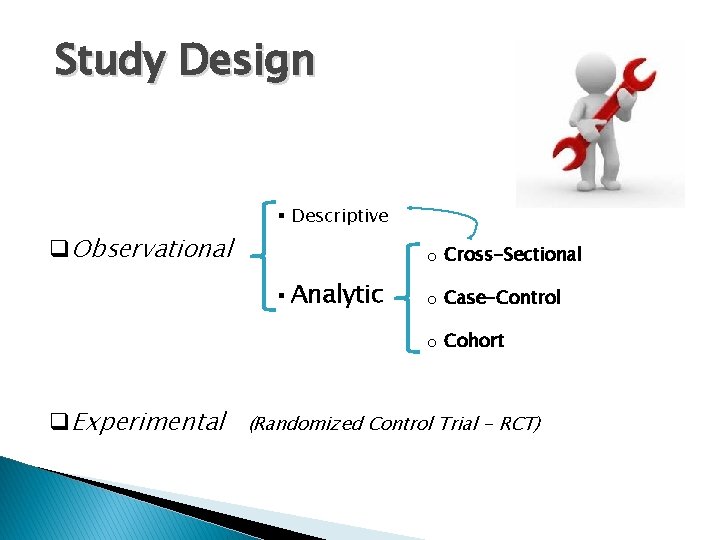
Study Design q. Observational § Descriptive o Cross-Sectional § Analytic o Case-Control o Cohort q. Experimental (Randomized Control Trial - RCT)

Descriptive Study � Case Report � Case series � Cross sectional � Longitudinal � Normative research � Secondary data analysis (summaries, meta-analysis) � Ecological Who? When? Where?

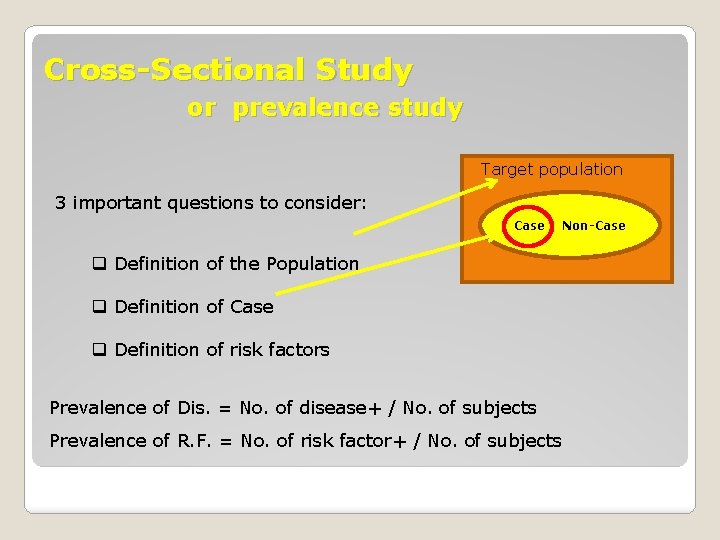
Cross-Sectional Study or prevalence study Target population 3 important questions to consider: Case Non-Case q Definition of the Population q Definition of Case q Definition of risk factors Prevalence of Dis. = No. of disease+ / No. of subjects Prevalence of R. F. = No. of risk factor+ / No. of subjects

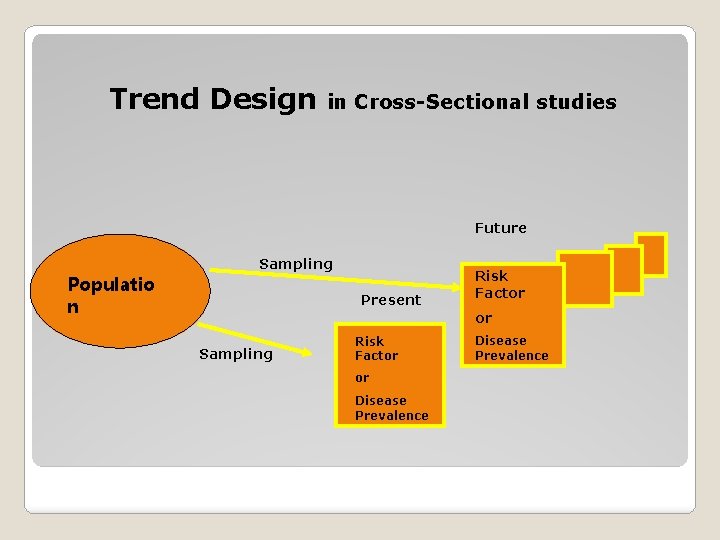
Trend Design in Cross-Sectional studies Future Populatio n Sampling Present Risk Factor or Sampling Risk Factor or Disease Prevalence

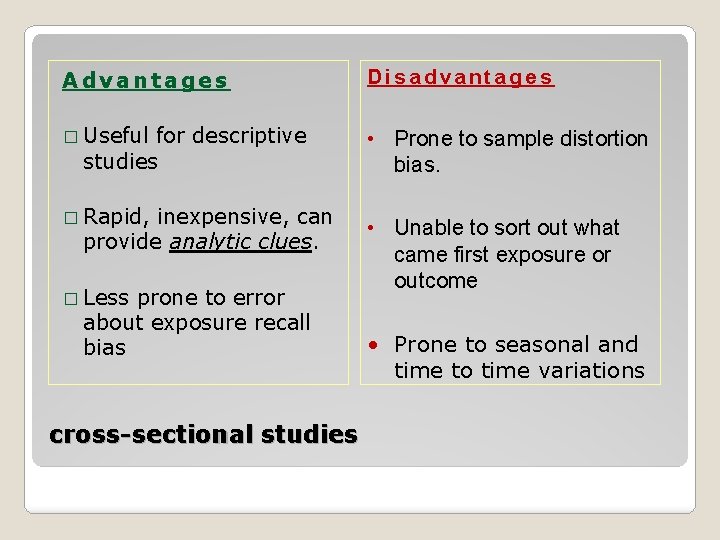
Advantages Disadvantages � Useful • Prone to sample distortion bias. for descriptive studies � Rapid, inexpensive, can provide analytic clues. � Less prone to error about exposure recall bias cross-sectional studies • Unable to sort out what came first exposure or outcome • Prone to seasonal and time to time variations

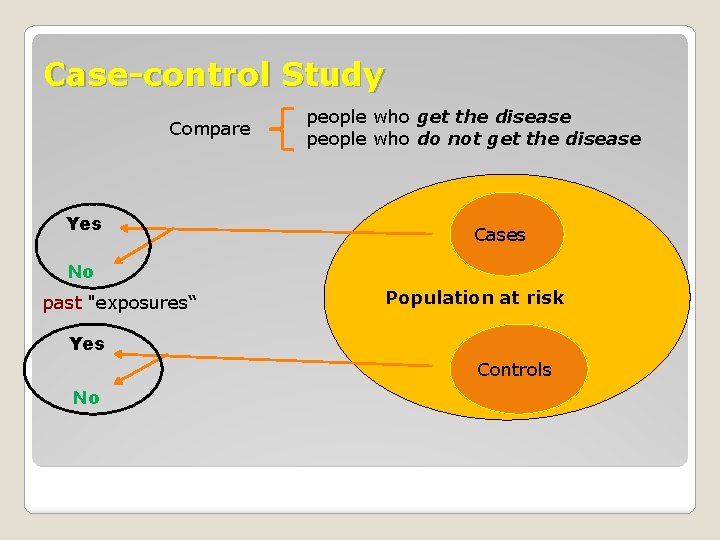
Case-control Study Compare Yes people who get the disease people who do not get the disease Cases No past "exposures“ Population at risk Yes Controls No

�Selection of cases t s r i F p e St v Precise definition of ‘case’. v Inclusion / Exclusion criteria. v How are cases to be identified? How recruited?

�Selection of Controls d n o c Se p e St q Source ( hospital patients without disease; neighborhood controls; random sample of population; sibs). q Inclusion / exclusion criteria. Controls must be related to the same population as the cases are.

�Collection of information rd i Th p e St Ø Identify risk factor of interest Ø Method of collection of information ( questionnaire; medical records; employment records) Ø Same procedure to be used for cases and controls Ø Interviewer should be unaware who is a case and who a control.

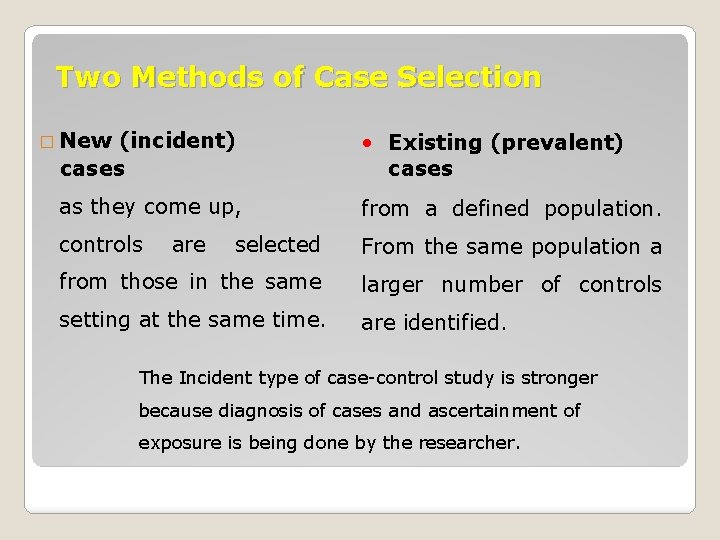
Two Methods of Case Selection � New (incident) cases • Existing (prevalent) cases as they come up, from a defined population. controls selected From the same population a from those in the same larger number of controls setting at the same time. are identified. are The Incident type of case-control study is stronger because diagnosis of cases and ascertainment of exposure is being done by the researcher.

ADVANTAGES � Relatively cheap compared to cohort studies � Relatively quick � Useful for study of rare diseases. � No ethical problems � Useful for diseases with long latent period. Case-control Studies Disadvantages • Estimate of disease incidence cannot be done • At times difficult to measure exposure accurately • Open to selection bias. • Difficult to interpret.

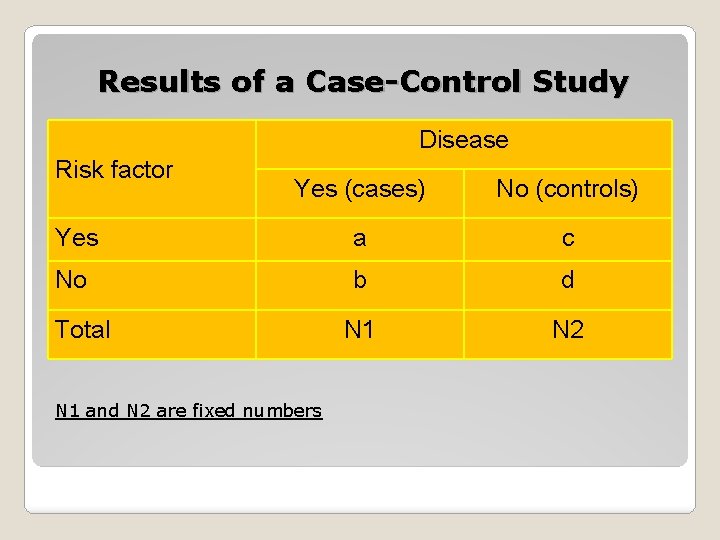
Results of a Case-Control Study Disease Risk factor Yes (cases) No (controls) Yes a c No b d N 1 N 2 Total N 1 and N 2 are fixed numbers

