RESEARCH ETHICS Dr Natasha Whiteman Department of Media


































- Slides: 35

RESEARCH ETHICS Dr Natasha Whiteman Department of Media and Communication University of Leicester new 9@le. ac. uk

Today • Why do we care about research ethics? • Key ethical principles in research • Implications of ethics for your own work.

What do we mean by research ethics? • Ethics – ethical principles as applied to the activity of research • Risk – i. e. risks of research activity – to safeguard the safety of yourself and others • Legal frameworks and codes of practice • Relevant to ALL researchers – across the sciences, social sciences and humanities.

Why behave ethically? • As researchers we have a responsibility to behave ethically (to be fair… to not cause harm to others. . etc) • To protect the rights of individuals, communities and environments involved in research • To assure favourable climate of public trust for continued research • To meet public demands for accountability and legal codes of responsible behaviour

Ethics in a changing academic context… • Contemporary codes of research ethics emerge from ethical frameworks developed to protect human subjects in biomedical research. • Increasing bureaucratisation and institutionalisation of research ethics in the social sciences • Move away from the expert authority of individual researcher • Introduction of ethics committees in UK higher education institutions, similar to Institutional Review Boards in the US • Increasing surveillance of research activity

“perverse consequences” of ethical regulation (Dingwall, 2008, 5)

What this means for you… • “Faculties and Departments are required to develop robust procedures for ensuring the ethical integrity of all student research (PGCE, MA, research degrees), all student research involving human participants being reviewed prior to the beginning of data collection and the results of student ethics reviews being reported to the Faculty Research Ethics Committee. ” http: //www. ioe. ac. uk/about/documents/About_Po licies/Researchethics. pdf

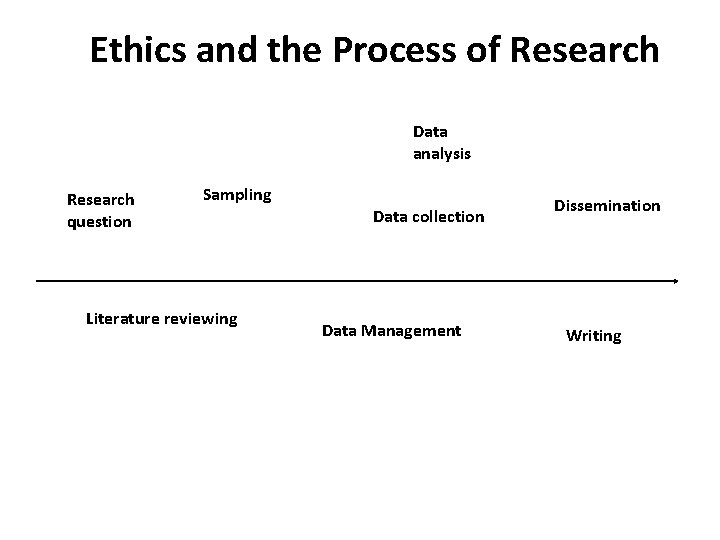
Ethics and the Process of Research Data analysis Research question Sampling Literature reviewing Data collection Data Management Dissemination Writing

To whom are we ethically responsible as researchers? • • • Our participants Our sponsors The University The general public Ourselves

Key terminology relating to research ethics • • • Human Subject Informed consent Right to Withdraw Confidentiality and Anonymity Disclosure

“Human participants” • “Faculties and Departments are required to develop robust procedures for ensuring the ethical integrity of all student research (PGCE, MA, research degrees), all student research involving human participants being reviewed prior to the beginning of data collection and the results of student ethics reviews being reported to the Faculty Research Ethics Committee. ” http: //www. ioe. ac. uk/about/documents/About_Po licies/Researchethics. pdf

• “The voluntary consent of the human subject is absolutely essential. This means that the person involved should have legal capacity to give consent; should be so situated as to be able to exercise free power of choice, without the intervention of any element of force, fraud, deceit, duress, over-reaching, or other ulterior form of constraint or coercion; and should have sufficient knowledge and comprehension of the elements of the subject matter involved, as to enable him to make an understanding and enlightened decision. This latter element requires that, before the acceptance of an affirmative decision by the experimental subject, there should be made known to him the nature, duration, and purpose of the experiment; the method and means by which it is to be conducted; all inconveniences and hazards reasonably to be expected; and the effects upon his health or person, which may possibly come from his participation in the experiment. • The duty and responsibility for ascertaining the quality of the consent rests upon each individual who initiates, directs or engages in the experiment. It is a personal duty and responsibility which may not be delegated to another with impunity. ” (1947 Nuremberg Code. )

What is a “human subject”? What is the difference between a “subject” and an “object”?

“Human Subjects” • “a living individual about whom an investigator conducting research obtains – data through intervention or interaction with the individual, or – Identifiable private information” (US Department of Health and Human services) • “‘Human participants’ (or subjects) are defined as including living human beings, human beings who have recently died (cadavers, human remains and body parts), embryos and foetuses, human tissue and bodily fluids, and human data and records (such as, but not restricted to medical, genetic, financial, personnel, criminal or administrative records and test results including scholastic achievements). (ESRC, 2005, 7)

Informed Consent • “Informed consent” refers to the idea that research participants should always be provided with enough information to make an informed decision about whether or not they want to take part in your study. • Consent to take part in a research project should be voluntary, informed and obtained without duress

Voluntary Informed Consent The Right to Withdraw • Participants have the right to withdraw from the project including after the data has been collected • They should be informed of this right • Any attempt to convince a participant not to withdraw should be carefully considered to ensure that no coercion or duress is being used • The possibility of a withdrawal after data collection should be considered in the planning stage to ensure that such an occurrence would not have a serious detrimental effect on the project

Voluntary Informed Consent • Questions to consider: – Is the participant competent to make their own decision regarding participation? – Are the participants free to choose to participate or not to participate? – Have the participants sufficient information to make this decision? – Do the participants fully understand this information?

Working with Children • Legal aspects: – Children defined as anyone under 18 years of age • One key ethical question: – From Whom do we obtain consent? • Child consent • Parental consent • Gatekeeper consent

Consent from Children • “In line with article 12 of the United Nations Convention on the Rights of the Child, which requires that children who are capable of forming their own views should be granted the right to express them freely in all matters affecting them, commensurate with their age and maturity, it is expected that efforts should be made to obtain informed consent from children involved in any research” “Research Concerning Children and Young People, ” http: //www 2. le. ac. uk/institution/committees/researchethics/research-concerning-children-and-young-peopleguidelines

When Might Informed Consent Not Be Appropriate? • • Observation in public places Research necessitating deception Covert research Using data in the public domain

Confidentiality and Anonymity • Confidentiality exists when only the researchers are aware of the participants’ identities and have promised not to reveal those identities to anyone else • Anonymity means that there is no way individual participants can be identified from any of the data or information collected from them in the research

Vidich and Bensman’s “ Springdale , New York” (1958) • Sociological study of social/political life in a small town “Springdale” (pseudonym) • Researchers ensured participants that their anonymity/privacy would be maintained. • Publication of Small Town in Mass Society (1958) resulted in anger/hostility because: Ø it was easy for those living in “Springdale” to identify “anonymised” participants Ø inhabitants were insulted by the researchers’ characterisations of the town and its people • RESULT: Refusal to co-operate with any social scientists in the future, no possibility of follow-up.

Data Protection Act (1998) • Relates to the processing of personal data (information) about individuals – Collecting data – only collect data that there is a legitimate research reason for collecting – “fair and for a specified purpose” – Handling data - Process and store data about individuals only with their consent – “Informed”consent – why collecting data? for what purpose? how will it be stored? who will have access? will it be published? – Disclosure - Do not disclose personal data about identified individuals to other people without their permission • Personal data on individuals should not be stored or archived any longer than is necessary for legitimate research reasons

Disclosure • On occasions a researcher may unexpectedly observe illegal behaviour or behaviour that is likely to be harmful to the participants or others • In these cases, the researcher must consider carefully whether to disclose this information to the appropriate authorities • In so far as possible, researchers should inform participants and/or their guardians of their intention to disclose and the reasons for this

ETHICAL CODES OF PRACTICE

“Commitment to a Code of Ethics will ensure that all research is conducted according to the following concerns: – To respect the autonomy of individuals – To avoid causing harm – To treat people fairly – To act with integrity – To use resources as beneficially as possible” http: //www. ioe. ac. uk/about/documents/About_Policies/Researchethics. pdf

Ethics guidelines • British Sociological Association “Statement of Ethical Practice” (2002) • British Educational Research Association “Ethical guidelines for educational research” (2004)

Carolyn Ellis “The Ethnographic I”

• How did reading this make you feel? Why? • What ethical issues does this reading raise? • Is this writing “ethical”?

IMPLICATIONS FOR YOUR WORK

What ethical issues might arise in your research? • Informed consent • Research relationships – Power dynamics in relationship between researcher and researched – Insider research (including conflict of interest/values) • Research topic/population – Sensitive topics – Vulnerable groups • Research Methods – Negotiating access (gatekeepers) – Covert observation/deception – Intrusive interventions – Risk of harm to participants (including stress, anxiety or humiliation) • Data collection, archiving and management – Confidentiality and data protection – Records of personal or confidential nature. • Dissemination and Publishing

TO FINISH…

Things to consider before and during research • Your own motivations – Eg how could your links to sponsors, personal convictions or career aspirations produce conflicts of interest? • Consent – Do you have informed consent? – Necessary elements: information; understanding; voluntariness; competence of potential participants; actual consent. • Confidentiality – Who has access to your data? How will it be stored? What will happen after research is completed? How are you going to inform your informants of confidentiality

Things to consider before and during research • Harm – Could your research cause physical, psychological, cultural, financial, legal or environmental damage? How easily can participants withdraw from the research after it begins? Have similar studies been done before? • Dissemination and feedback – Are results available and comprehensible to participants? Could they be misinterpreted or misused? Who ‘owns’ the results? Your sponsor? • Cultural awareness – Have you considered the personality, rights, beliefs and ethical views of your researched individuals/communities? • Would you wish to be treated as you are treating your research participants?

Dr Natasha Whiteman new 9@le. ac. uk
 Natasha whiteman
Natasha whiteman Julia whiteman
Julia whiteman Elizabeth whiteman md
Elizabeth whiteman md I consider your behavior rude irresponsible and offensive
I consider your behavior rude irresponsible and offensive Twh family health team
Twh family health team Natasha hutson
Natasha hutson Natasha weaver
Natasha weaver Natasha duffy
Natasha duffy Natasha alechina
Natasha alechina Natasha alechina
Natasha alechina Natasha tierney
Natasha tierney Natasha bowler
Natasha bowler Natasha milashevich
Natasha milashevich Natasha shetty
Natasha shetty Natasha ray
Natasha ray Natasha webb prather
Natasha webb prather Tatiana natasha toporcov
Tatiana natasha toporcov Natasha grech
Natasha grech Natasha werpy
Natasha werpy Natasha st cyr
Natasha st cyr Natasha ayres
Natasha ayres Natasha larocque
Natasha larocque Natasha niemi
Natasha niemi Natasha tracy
Natasha tracy Natasha gerolami
Natasha gerolami Natasha sharygina
Natasha sharygina Natasha lee muscle
Natasha lee muscle Natasha jankowski
Natasha jankowski Descriptive ethics
Descriptive ethics Realism vs anti realism
Realism vs anti realism Macroethics definition
Macroethics definition Aspects of honesty
Aspects of honesty Meta ethics vs normative ethics
Meta ethics vs normative ethics Descriptive ethics vs normative ethics
Descriptive ethics vs normative ethics Beneficence examples
Beneficence examples Meta ethics vs normative ethics
Meta ethics vs normative ethics