Research article structure Where can reporting guidelines help








- Slides: 16

Research article structure: Where can reporting guidelines help? Iveta Simera The EQUATOR Networkshop

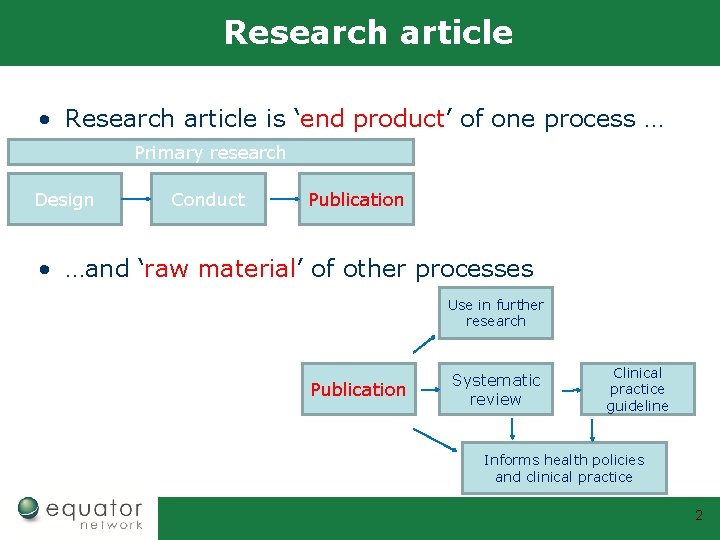
Research article • Research article is ‘end product’ of one process … Primary research Design Conduct Publication • …and ‘raw material’ of other processes Use in further research Publication Systematic review Clinical practice guideline Informs health policies and clinical practice 2

Research article: “fit for purpose” • Published research article is a permanent record • Will be used by different users for different purposes which means different needs for reporting – From brief scanning for information – To rigorous scrutiny of methodology and findings for possible comparison across studies in systematic reviews • Published article should be fit for these multiple purposes • New ways of publishing (e. g. online suppl) can aid readability without excluding crucial information 3

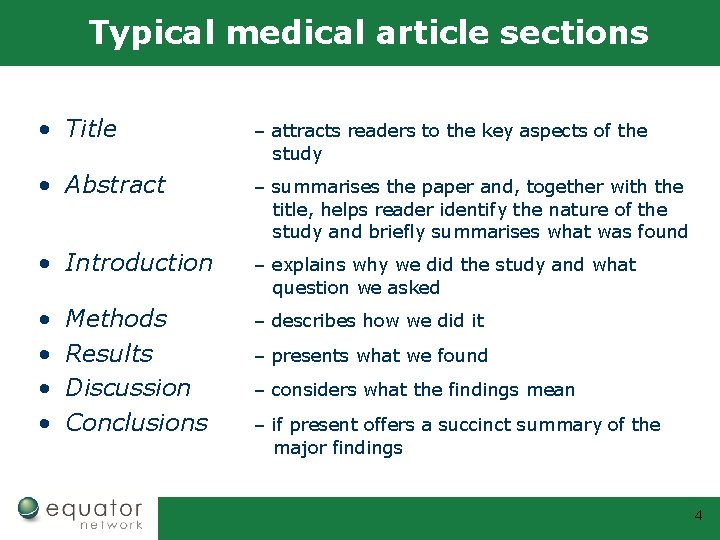
Typical medical article sections • Title – attracts readers to the key aspects of the study • Abstract – summarises the paper and, together with the title, helps reader identify the nature of the study and briefly summarises what was found • Introduction – explains why we did the study and what question we asked • • – describes how we did it Methods Results Discussion Conclusions – presents what we found – considers what the findings mean – if present offers a succinct summary of the major findings 4

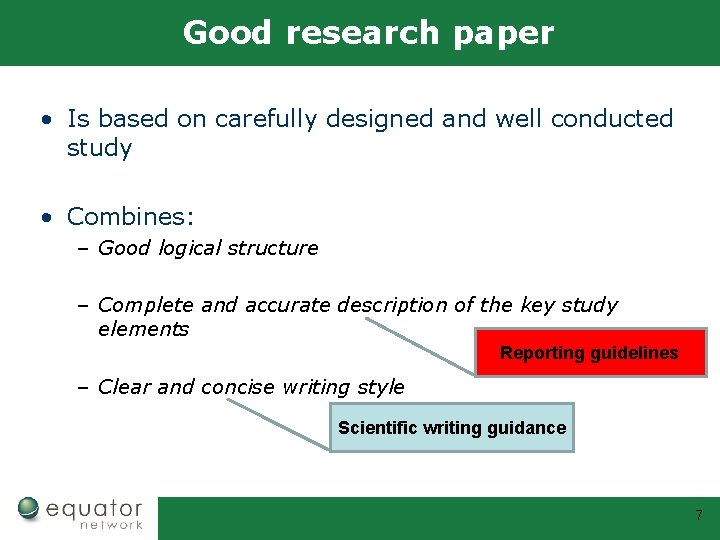
Good research paper • Is based on carefully designed and well conducted study • Combines: – Good logical structure – Complete and accurate description of the key study elements – Clear and concise writing style 5

Good research paper • Is based on carefully designed and well conducted study • Combines: – Good logical structure – Complete and accurate description of the key study elements – Clear and concise writing style Scientific writing guidance 6

Good research paper • Is based on carefully designed and well conducted study • Combines: – Good logical structure – Complete and accurate description of the key study elements Reporting guidelines – Clear and concise writing style Scientific writing guidance 7

Reporting guidelines (RGs) • Focus on scientific content of the article • Provide structured advice on what to include in a research report • Definition: – Specify a minimum set of items required for a clear and transparent account of what was done and what was found in a research study, reflecting in particular issues that might introduce bias into the research – Form: often as a checklist (perhaps also a flow diagram) • Most internationally accepted RGs – Based on evidence – Consensus of relevant stakeholders (multidisciplinary group) Moher et al. PLo. S Med 2010 8

Reporting guidelines database 9

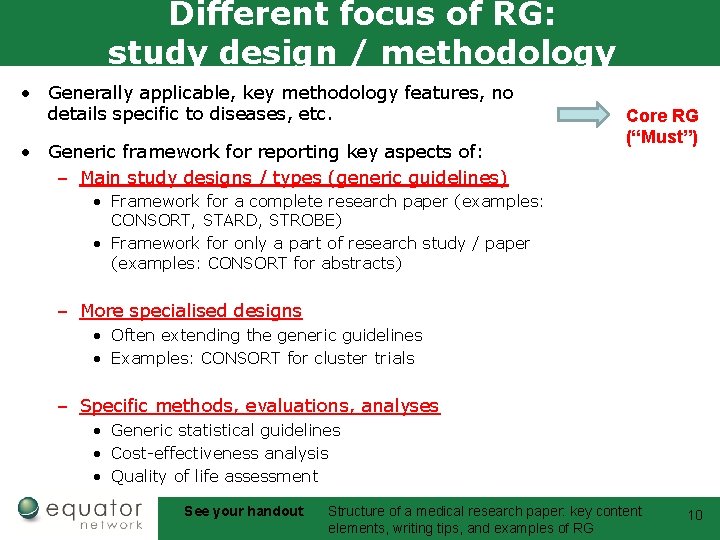
Different focus of RG: study design / methodology • Generally applicable, key methodology features, no details specific to diseases, etc. • Generic framework for reporting key aspects of: – Main study designs / types (generic guidelines) Core RG (“Must”) • Framework for a complete research paper (examples: CONSORT, STARD, STROBE) • Framework for only a part of research study / paper (examples: CONSORT for abstracts) – More specialised designs • Often extending the generic guidelines • Examples: CONSORT for cluster trials – Specific methods, evaluations, analyses • Generic statistical guidelines • Cost-effectiveness analysis • Quality of life assessment See your handout: Structure of a medical research paper: key content elements, writing tips, and examples of RG 10

Different focus of RG: specific discipline / clinical area • Key focus is on discipline / clinical area specific issues – Different ‘degree’ of specificity • May or may not address general methodology items Should be used with relevant generic methodology guidelines as they often focus only on content specifics • May focus on a complete research study / paper or only on a part • Examples – RCTs in leukaemia; longitudinal studies in rheumatology – Economic evaluations in obstetrics See your handout: Structure of a medical research paper: key content elements, writing tips, and examples of RG 11

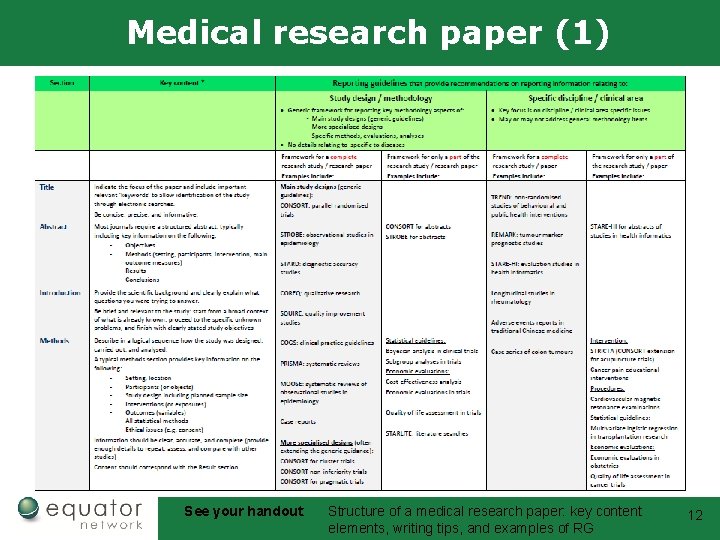
Medical research paper (1) See your handout: Structure of a medical research paper: key content elements, writing tips, and examples of RG 12

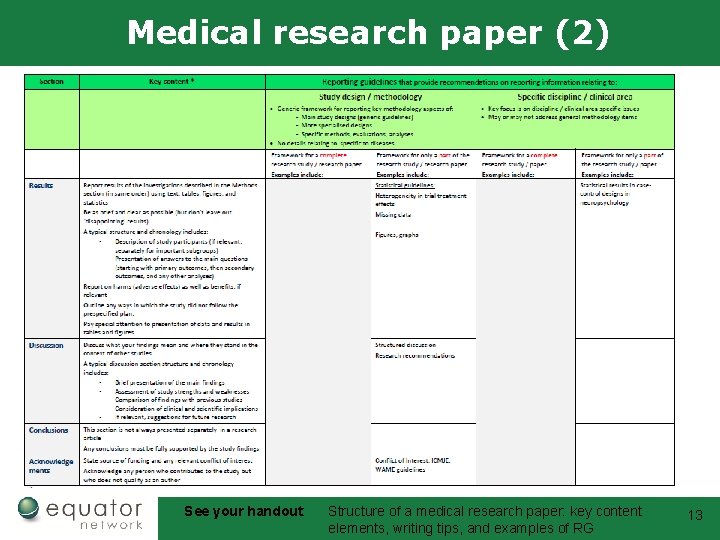
Medical research paper (2) See your handout: Structure of a medical research paper: key content elements, writing tips, and examples of RG 13

Common problems in research reporting Methods and Results – Non-reporting or delayed reporting of whole studies – Omissions or misinterpretation of results in abstracts – Omission of crucial information in the description of research methods and interventions – Inconsistencies between study protocol (or register) and publication – Incomplete reporting (data cannot be included in SR / MA) – Selective reporting of only some outcomes or analyses – Inadequate reporting of harms – Inadequate statistical reporting – Confusing or misleading presentation (e. g. presenting data & graphs in confusing or misleading ways - particularly important for presenting benefits and harms) – General misinterpretation of study findings (spin) 14

Responsibilities of researchers / authors Key principles for responsible research reporting • The research being reported should have been conducted in an ethical and responsible manner and should comply with all relevant legislation. • Researchers should present their results clearly, honestly, and without fabrication, falsification or inappropriate data manipulation. • Researchers should strive to describe their methods clearly and unambiguously so that their findings can be confirmed by others. • Researchers should adhere to publication requirements that submitted work is original, is not plagiarised, and has not been published elsewhere. • Authors should take collective responsibility for submitted and published work. • The authorship of research publications should accurately reflect individuals’ contributions to the work and its reporting. • Funding sources and relevant conflicts of interest should be disclosed. Reproduced from the International standards for authors of scholarly publications (http: //publicationethics. org/international-standards-editors-and-authors) 15

How I can improve reporting of my research study • Find out about reporting requirements early, when planning your research study • When writing up your research, check the EQUATOR website for any new relevant guidelines to help improve the quality of your manuscript • Adhere to the relevant reporting guideline(s). • When not reporting on certain items explain why. • Remember that reporting guidelines provide a minimum set of items; other details specific to your particular study might be relevant for a clear and complete account of what was done and found (consider in particular items that might have introduced bias into your research). • It is important to provide enough information to allow your study to be potentially reproducible by others. • See ‘Steps to consider’ in Simera et al. BMC Medicine 2010, 8: 24 16