Research and regulation Sandy Mather Director of Regulation






















































- Slides: 57

Research and regulation Sandy Mather Director of Regulation

Aims l l To explain the HTA’s structure, ethos and functions To explain the legislative framework for licensing and inspection To summarise our standards for licensing and explain compliance reporting To explain the role of the Designated Individual, Licence Holder and Persons Designated

Outcomes l Delegates should have a greater understanding of l l l the HTA’s structure, ethos and functions how the HT Act affects researchers HTA standards for licensing, inspection and compliance reporting the role of the Designated Individual, Licence Holder and Persons Designated HTA will have a greater understanding of l l the issues which effect the research community the practical implications of being a Designated Individual or Person Designated

Structure, ethos and functions

HTA - structure l Human Tissue Authority - members l l 17 (including the Chair) Lay majority Professional representatives Human Tissue Authority – staff l l 20 (including Chief Executive) Four directorates – regulation, policy, communication and resources

The HTA’s regulatory aim To create an effective regulatory framework for the removal, retention, use and disposal of human tissue and organs in which the public and professionals have confidence

What we are l l l An independent regulator Inspiring professional, patient and public confidence A proportionate regulator l l Inspecting according to risk Flexible Supportive Collaborating with other regulators l Best practice and avoid duplication

How we will do this l l l By consulting widely and listening By providing clear guidance and advice Keeping things as uncomplicated as possible

How will we license and inspect? l l l Compliance reporting HTA evaluate evidence in compliance report Inspect according to risk

Inspection l l Inspection process includes site visits and desk-based evaluation of information Site visits l carried out prudently to benefit both applicant and the regulator l gather additional visual and aural evidence l test compliance with standards l test validity of risk assessment and evidence provided

Licensing storage of human tissue for research activities

The legislative framework l l The HT Act and EU Tissues and Cells Directive Two stages of commencement for licensing April 2006 – establishments storing tissue for human application September 2006 – all other sectors l l Storage for research Anatomical examination Public display Pathology

The HT Act – S 16 16(1) No person shall do an activity to which this section applies otherwise than under the authority of a licence granted for the purposes of this section. 16 (2)(e) the storage of (i) the body of a deceased person, or (ii) relevant material which has come from a human body for use for a scheduled purpose

HT Act l The HT Act makes consent the fundamental principle l l Storage and use of body parts, organs and tissue from the living or deceased for specified purposes Removal of material from the deceased

Licensing l l One activity per licence A licence must specify the premises where the activity is to be carried out A licence cannot authorise licensed activity on premises at different places One person (Designated Individual) supervises the activities under a licence

Research

Research - will I need a licence? Q – Do I undertake research on tissue samples from living patients? l Tissue removed and stored for the primary purpose of diagnosis or treatment l l No licence Tissue removed and stored for the primary purpose of research l l l Distribution to other researchers (tissue bank) – licence A specific research project with ethical approval – no licence A possible project in the future – licence

Research – will I need a licence? Q – Do I undertake research on tissue samples from deceased patients? l Tissue removed and stored to determine the cause of death l l Licence required Tissue removed and stored for the primary purpose of research l l l Distribution to other researchers (tissue bank) – licence A specific research project with ethical approval – no licence A possible project in the future – licence

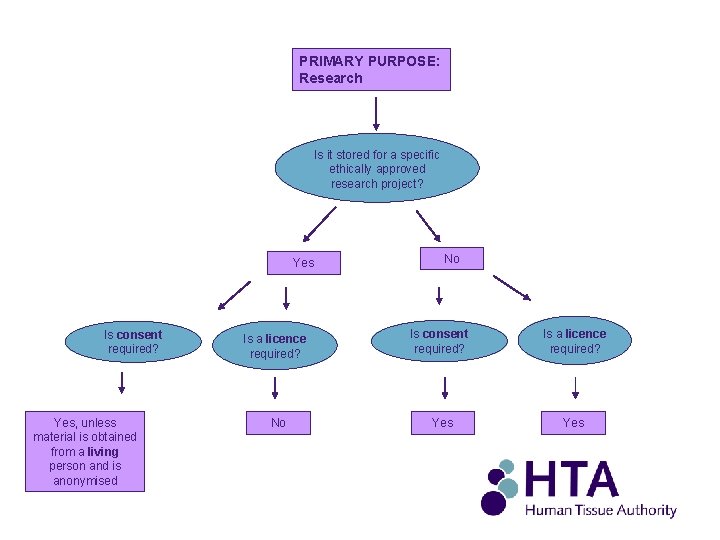
PRIMARY PURPOSE: Research Is it stored for a specific ethically approved research project? Yes Is consent required? Yes, unless material is obtained from a living person and is anonymised No Is a licence required? Is consent required? Is a licence required? No Yes

The licensing process

Overview of the licensing process l l l l l Role of Designated Individual and Licence Holder Compliance report, standards and guidance notes On-line application Deemed licences Licensing database Evaluating licence applications Licensing Panels Issuing licences Representations and appeal

The Designated Individual

The role of the Designated Individual (DI) l l l Specific responsibilities as set out in section 18 of the Human Tissue Act The DI is the person under whose supervision the licensed activity/ies are authorised to be carried on HT Act does not state who should be a DI

The role of the DI – Lord Warner’s view l ‘The person might be a head of department, a clinician, a scientist, or a manager. What is important is that it is a person who is in a position to secure that activities are conducted properly by people who are suitable to carry out those activities and that all the necessary requirements are complied with’ l Lord Warner – House of Lords – Grand Committee

The role of the DI – HTA’s view l l DI has to have knowledge and understanding of the HT Act and the relevant Codes of Practice DI has to demonstrate managerial capability, ensuring development and implementation of quality management systems and be able to effect change Links to board level Have time within substantive role to carry out responsibilities and ensure compliance with licensing conditions

The role of the DI – the sectors’ views l l Varying views about who should be the DI across 5 sectors Varying organisational structures Concern about level of responsibility We found that licence applicants tended to have a limited understanding of l l l Duty of ‘Designated Individual’ (Section 18) Role of ‘Licence holder’ (Para 6(4) of Schedule 3) Role of ‘Designated Persons to whom a licence applies’ (Section 17)

The role of the DI – set out in HT Act l Designated Individual l Person under whose supervision the licensed activity is authorised to be carried on Must consent to an application or make it himself Has statutory duties as set out in Section 18 - that the other persons to whom the licence applies are suitable persons to participate in the carrying-on of the licensed activity - that suitable practices are used in the course of carrying on that activity and - that the conditions of the licence are complied with

The role of the DI – set out in HT Act l l l Securing compliance with licence conditions Licence conditions can be statutory, standard and additional Examples of statutory licence conditions – the “givens” l l l The licensed activity must only take place on the premises specified in the licence “Supervise” activities carried on under a licence Record information as required by HTA (direction making powers) Keep records specified by HTA as required by HTA Provide copies of records or extracts to HTA as may be specified Pay fees to the Authority in respect of its costs in “superintending compliance with the terms of licences”

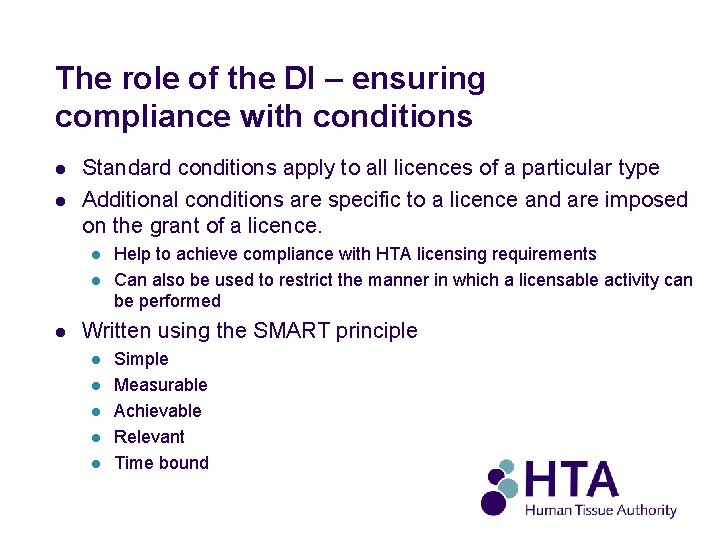
The role of the DI – ensuring compliance with conditions l l Standard conditions apply to all licences of a particular type Additional conditions are specific to a licence and are imposed on the grant of a licence. l l l Help to achieve compliance with HTA licensing requirements Can also be used to restrict the manner in which a licensable activity can be performed Written using the SMART principle l l l Simple Measurable Achievable Relevant Time bound

HTA Governance framework l l Various models of governance HTA aims to explain the roles and responsibilities Provide education and training via workshops E-learning packages

HTA Governance Framework l l Designated Individual Licence applicant (if different to DI) Person Designated as a person to whom the licence applies Persons acting under the direction of a DI or Person Designated by DI

Governance l Licence holder (if different to DI) l l l Must have consent of DI for application Can apply to vary licence to remove DI without his/her consent Can be a corporate body eg NHS trust

Governance l Person designated as a person to whom the licence applies l l l Persons acting under the direction of a DI or Person Designated l l Must be named in a notice given by the DI to the Authority Other people can work under the direction of this person Any person Responsible Person under the EUTCD l Must have scientific or medical experience

HTA framework - based on governance of institution Designated Individual Person Designated by DI Licence Holder (if not DI) Other people working on licensed premises and carrying out licensed activities do so under the direction of the Designated Individual or a Person Designated by the DI

Role of the Authority l l Pre-conditions to the grant of a licence The Authority must be satisfied that l l l The proposed DI is a suitable person to supervise the activity to be authorised by the licence The applicant is a suitable person to be the holder of the licence The premises are suitable for the activity to be authorised by the licence A copy of the conditions to be imposed by the licence must be acknowledged in writing by the applicant for the licence and where different the proposed DI Compliance report and application form enable the Authority to determine “suitable”

DI training days l l l l 15 June – Liverpool 19 June – London 5 July – Bristol 19 July – London 9 August – Birmingham January 2007 – tbc Email di@hta. gov. uk

Compliance reporting

Compliance report and guidance notes l Compliance report l l l l Establishment Information Designated Individual Licence holder (corporate or individual) Consent – 3 standards Governance and Quality Systems – 8 standards Premises, Facilities and Equipment – 5 standards Disposal – 2 standards Guidance l l l Website Licensing FAQs A guide to licensing for DIs and Licence Holders Application guidance Telephone and email advice

The Compliance Report l l Standards in a tabulated format On-line document Narrative and numerical scoring Licence applicant and Designated Individual complete jointly with team

Standards l l l l Generic Goal to be achieved Assess compliance with the Act and Codes of Practice Evidence of compliance l l Sector specific Activities that are needed to achieve the standard

Compliance Report l Purpose l l l Allows applicant to reflect on progress against standards Enables an evaluation of suitability for a licence Advantages l l l Engenders change Drives up standards Empowers regulated sector Identifies areas for improvement Implements changes to achieve them

On line application

Applications for licences l Storage of tissue for human application l l Licence will be for storage for transplantation Storage of tissue for research l Licence will be for storage for use for a Scheduled Purpose other than transplantation




Deemed licences

Issuing deemed licences l l Applications to be received by mid August 2006 HTA check contact details and issue deemed licence by 1 September 2006 Customer satisfaction questionnaire included Data transferred from website to licensing database for HTA evaluation

Evaluating licence applications

How the compliance report is evaluated l Numerically l l l Qualitatively l l Scale of 1 -4 1 – Standard not met 4 – Standard fully met Scores are not cumulative Narrative to support the self-assessed score by the applicant Compliance report is evaluated by the regulator l l Regulator awards a score based on an evaluation of the available information about an applicant Regulator allocates a final score taking account of the applicant’s self-assessment score

Licensing Panels

Licensing panels l Two panels working on a fortnightly rotation – Panel A and B l l l Each panel comprises two members of staff Chaired by a manager Escalate if there is disagreement or legal advice may be needed l l Director of Regulation or other member of SMT joins the panel and takes over the Chair (has casting vote) Legal advisor may be engaged

Making judgements about “suitability” as per the HT Act l Licensing Panel to decide l l l l l Whether satellite arrangements are suitable If the proposed DI is suitable If the licence holder is suitable If the premises are suitable for the proposed activity Whether suitable practices take place at those premises Whether to apply conditions Whether to grant a licence The duration of the licence To refuse to grant a licence

Issuing licences

Two documents l Letter to offer licence l l Sent from chair of Licensing Panel Includes guidance about the right to make representations Requirement to acknowledge licence in writing Licence with four annexes l l Additional conditions (plus reasons)– specific to application Standard conditions – sector specific and imposed by HTA Statutory conditions – sector specific and as set out in the HT Act Definition of terms

Summary l HTA’s approach to regulation l l How we will licence the research sector To explain the role of the Designated Individual, Licence Holder and Persons Designated Compliance reports l l l Flexibility, advice and guidance, Developed standards with each sector Applicant critically reviews own progress against standards Licensing storage of tissue for research from 1 September 2006

www. hta. gov. uk
 Sandy mather
Sandy mather Danny mather warrington
Danny mather warrington Nadine mather
Nadine mather Maria marcinek
Maria marcinek W.w. norton
W.w. norton Sandy feels dirty unless she bathes and changes
Sandy feels dirty unless she bathes and changes Andy sometimes comics. (to read)
Andy sometimes comics. (to read) Sandy tatiana rivas
Sandy tatiana rivas Sandy miller real estate
Sandy miller real estate Sandy irani
Sandy irani Beaches near me
Beaches near me Ram singh chauhan age
Ram singh chauhan age Sandy soil characteristics
Sandy soil characteristics Sandy kaul
Sandy kaul Sandy hui
Sandy hui Arthur tress fish tank sonata
Arthur tress fish tank sonata Sociloga
Sociloga Cliffs
Cliffs Sandy bertrand
Sandy bertrand Sandy biondo
Sandy biondo Mmiccs
Mmiccs Hyrricane sandy
Hyrricane sandy Sandy hampel
Sandy hampel Sandy elliott
Sandy elliott Prove by exhaustion
Prove by exhaustion Sandy washburn
Sandy washburn Sandy kutin
Sandy kutin Sandy myles
Sandy myles The probability of sandy flipping
The probability of sandy flipping Sandy mazzola
Sandy mazzola Hurrricane sandy
Hurrricane sandy Sandy shurin
Sandy shurin Sandy
Sandy Sandy hillman
Sandy hillman Sandy papadopoulos
Sandy papadopoulos Alice kutin
Alice kutin Sandy hampel
Sandy hampel Hyposecretion of growth hormone
Hyposecretion of growth hormone Sandy autard
Sandy autard Sandy lopez biografia
Sandy lopez biografia Sandy bertrand
Sandy bertrand Sslh uci
Sslh uci Alex sandy pentland
Alex sandy pentland Sandy bobek
Sandy bobek Sandy madrid
Sandy madrid Matthew som sandy crossboss
Matthew som sandy crossboss Sandy antunes
Sandy antunes English comic actor and film director
English comic actor and film director English comic actor and film director
English comic actor and film director English comic actor and film director
English comic actor and film director The text-based director, also known as the
The text-based director, also known as the Deberes de un director de escuela
Deberes de un director de escuela Program vizita penitenciar miercurea ciuc
Program vizita penitenciar miercurea ciuc Peranan pengarah syarikat
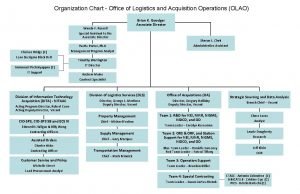
Peranan pengarah syarikat Logistics organizational chart
Logistics organizational chart Director comercial turistico tenerife
Director comercial turistico tenerife Director vs producer
Director vs producer Salaam bombay! nominations
Salaam bombay! nominations