Representing Molecules Resonance Exceptions to the Octet Rule








- Slides: 14

Representing Molecules Resonance Exceptions to the Octet Rule Formal Charge

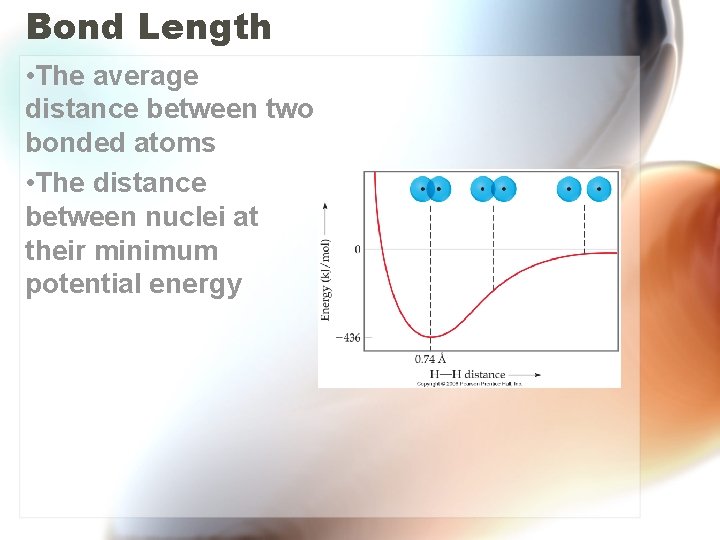
Bond Length • The average distance between two bonded atoms • The distance between nuclei at their minimum potential energy

Bond Dissociation Energy • Also called “bond energy” • The energy required to break a bond and form neutral isolated atoms

Trends in Bond Energy • C-C vs. C=C vs. C≡C – Higher bond energy for multiple bonds compared to single bonds • Triple bonds are shorter on average than single bonds – There can be a lot of variation in bond length, depending on other bonds for those atoms

Bond Energy & Bond Length • Higher bond dissociation energy is linked to lower chemical reactivity • Longer bond lengths lower bond dissociation energies – “Short bonds are strong bonds”

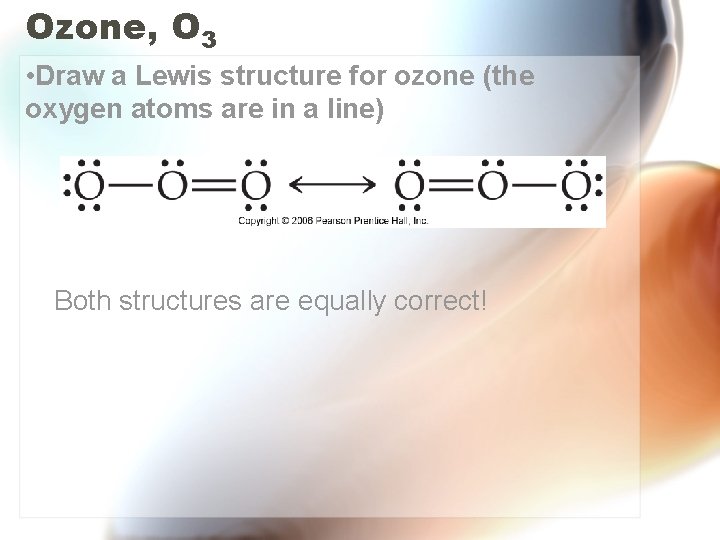
Ozone, O 3 • Draw a Lewis structure for ozone (the oxygen atoms are in a line) Both structures are equally correct!

Resonance Structures • Sometimes, more than one correct Lewis structure can be drawn for a compound – A single Lewis structure may be inadequate for describing the situation – “resonance” structures • Same arrangement of atoms, same numbers of single and multiple bonds • Link the multiple structures with a double headed arrow

Resonance • The actual structure is a “blend”, somewhere in between the Lewis structures • Alternating single/double bonds would have different bond lengths – In the real molecule, all the bond lengths are identical!

Problem • A problem to try: – Draw resonance structures for the nitrate ion, NO 3 -

Exceptions to the Octet Rule • Elements in row 3 or below on the periodic table can have expanded octets – i. e. , they can have more than 8 electrons, because they may use d orbitals in bonding • B, Be may be “electron deficient” – Fewer than 8 electrons around central atom

Nonequivalent Lewis Structures • If a molecule or polyatomic ion exceeds the octet rule, or if different skeletal arrangements (i. e. isomers) are possible, non-equivalent Lewis structures may be drawn. • Million dollar question: Which Lewis structure best describes the actual bonding situation? – Use “formal charge” to evaluate

Formal charge • FC = # valence electrons – (# of lone pair electrons) – (# bonds) – Calculated for each atom – Sum of formal charges must equal overall charge of species • The most appropriate Lewis structures have: – Lowest formal charges (zero is best) – Negative formal charge on the most electronegative element

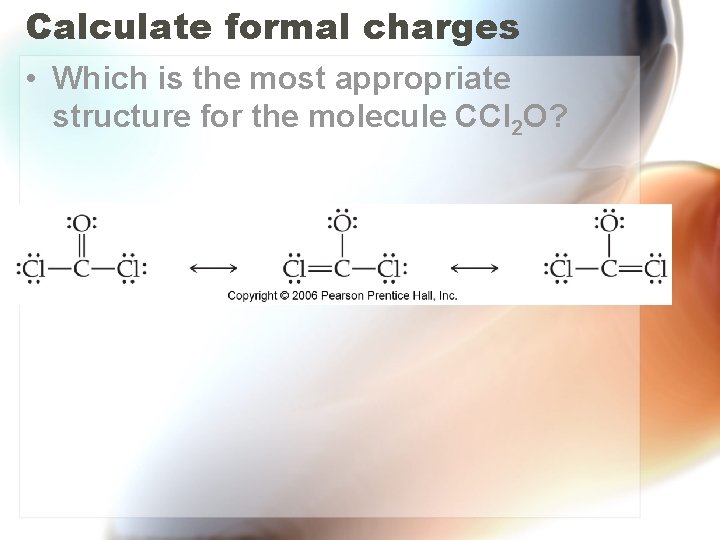
Calculate formal charges • Which is the most appropriate structure for the molecule CCl 2 O?

Problem • Below are two different Lewis structures for nitrous acid (HNO ). 2. . H-O-N=O: . . . H-N=O. . : O: . . • Which is the better Lewis structure based only on formal charge arguments? . .