Replacement Reactions What happens to ionic compound in











- Slides: 19

Replacement Reactions

What happens to ionic compound in water?

Ionic Compounds �When ionic compounds dissolve they become aqueous �The ion separate and are free to move about in the water �The separation of ions is very important to remember

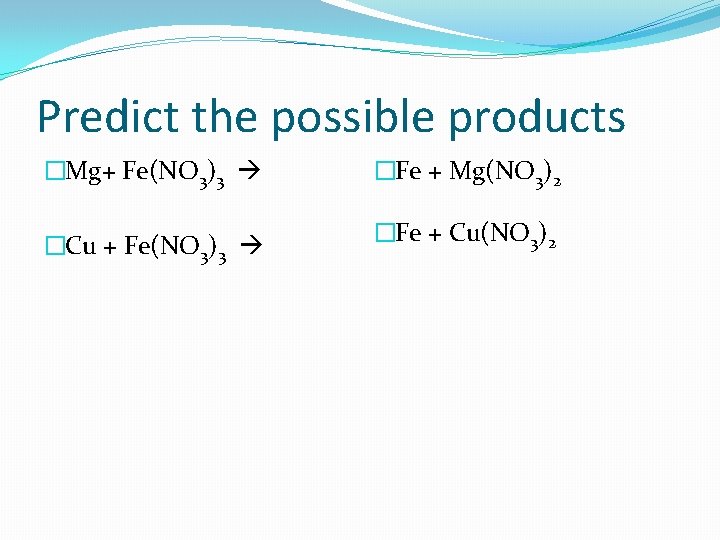
Predict the possible products �Mg+ Fe(NO 3)3 �Cu + Fe(NO 3)3 �Fe + Mg(NO 3)2 �Fe + Cu(NO 3)2

Lets see what happens �Mg+ Fe(NO 3)3 �Cu + Fe(NO 3)3 �Fe + Mg(NO 3)2 �No reaction

Why did this happen �In order for a single replacement reaction to occur the element must be more reactive than the element that it will replace �Mg is more reactive than Fe, so we saw a reaction �Cu is less reactive than Fe, so we saw no reaction

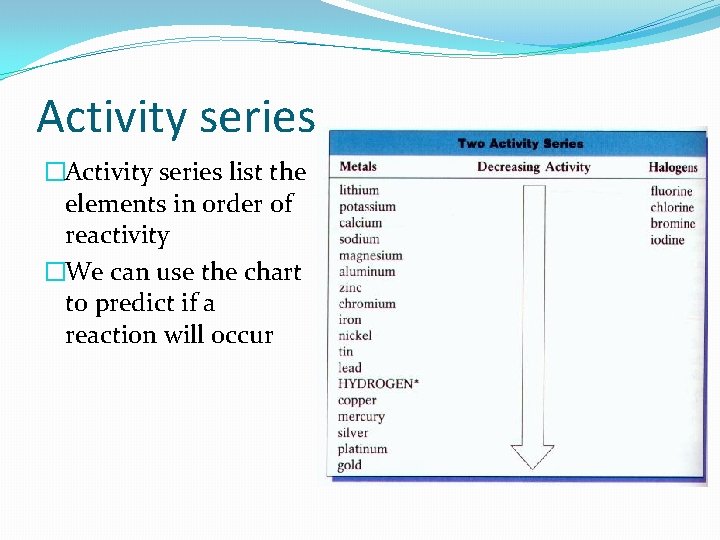
Activity series �Activity series list the elements in order of reactivity �We can use the chart to predict if a reaction will occur

Double replacement reactions �Two ionic compounds react and for two new ionic compounds �In order for a DR reaction to occur one of the new compounds formed must be �A solid �A gas �Water

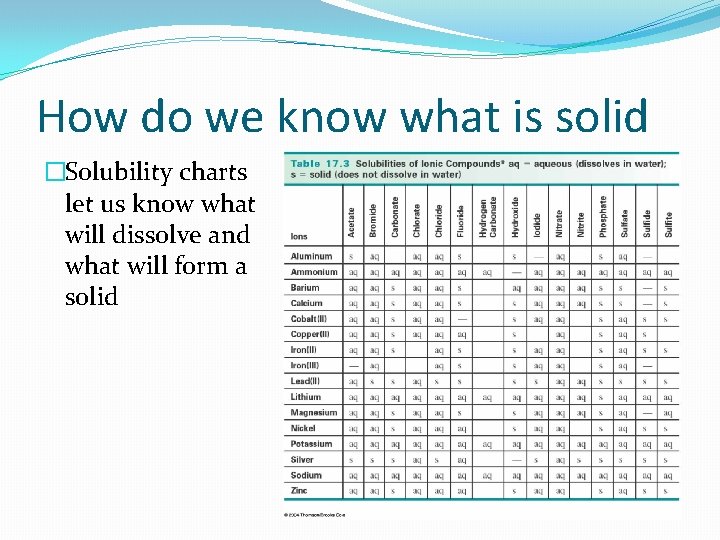
How do we know what is solid �Solubility charts let us know what will dissolve and what will form a solid

Lets do an example �What will happen when we mix solutions of silver nitrate and sodium chloride �Ag. NO 3 + KCl �The “possible products are …. . �Ag. NO 3 + KCl Ag. Cl + KNO 3 �Is either one a solid? �Yes, Ag. Cl is insoluble, so it is solid

Lets do another example �What will happen when you mix solutions of potassium chloride and copper II nitrate �KCl + Na. NO 3 �The possible products are…. �KCl + Cu(NO 3)2 Na. Cl and KNO 3 �Are either the products solids �Neither products are solids they are both aqueous �So…… No reaction

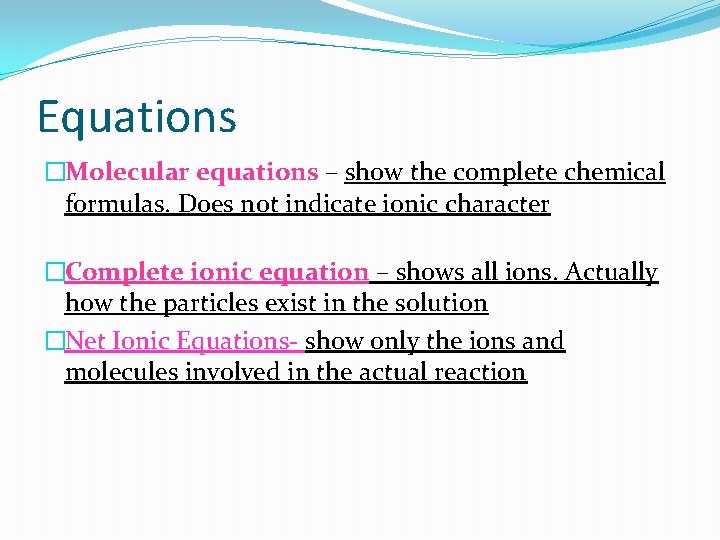
Equations �Molecular equations – show the complete chemical formulas. Does not indicate ionic character �Complete ionic equation – shows all ions. Actually how the particles exist in the solution �Net Ionic Equations- show only the ions and molecules involved in the actual reaction

Rules �When writing ionic equations, you must remember that solids, gases or water do not dissociate �Spectator ions – ions that appear on both sides of the equation. They have very little to do with the chemical reaction

Steps for Writing Ionic Equations Know these steps Write the balanced molecular equation (balanced chemical equation) 2. Break every thing down into its ions EXCEPT the solid, gas, water, or(complete ionic equation) 3. Cross out everything that is the same on both sides (spectator ions) 4. Write what is left (net ionic equation) 1.

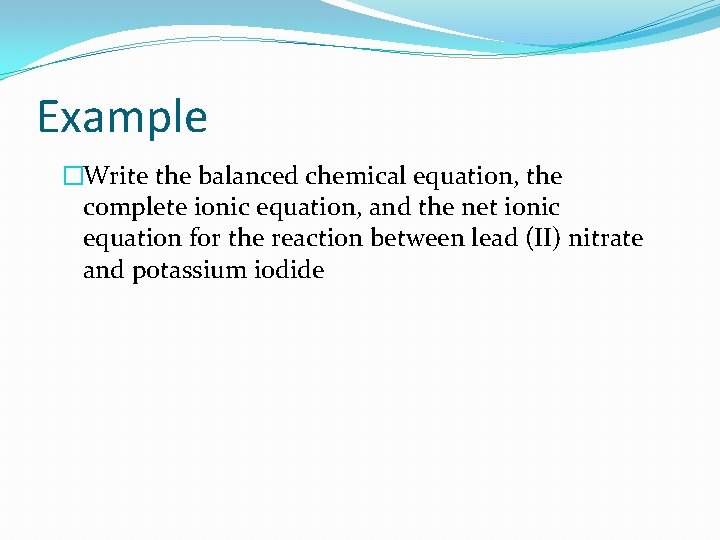
Example �Write the balanced chemical equation, the complete ionic equation, and the net ionic equation for the reaction between lead (II) nitrate and potassium iodide

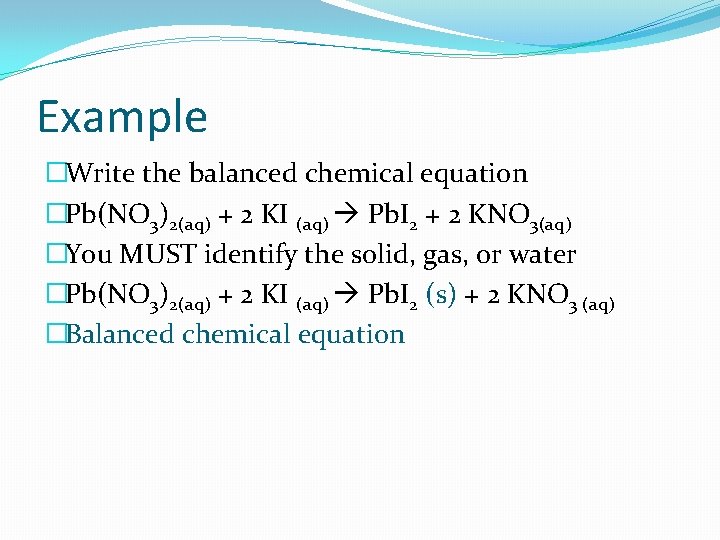
Example �Write the balanced chemical equation �Pb(NO 3)2(aq) + 2 KI (aq) Pb. I 2 + 2 KNO 3(aq) �You MUST identify the solid, gas, or water �Pb(NO 3)2(aq) + 2 KI (aq) Pb. I 2 (s) + 2 KNO 3 (aq) �Balanced chemical equation

Example �Now break every thing except the solid, gas, or water into its ions �Remember ions are things with charges �Everything will be broken down into one positive charge and one negative charge

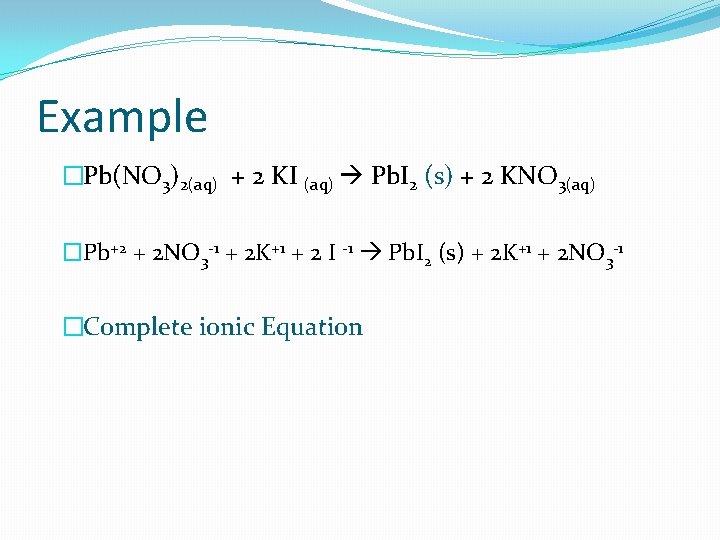
Example �Pb(NO 3)2(aq) + 2 KI (aq) Pb. I 2 (s) + 2 KNO 3(aq) �Pb+2 + 2 NO 3 -1 + 2 K+1 + 2 I -1 Pb. I 2 (s) + 2 K+1 + 2 NO 3 -1 �Complete ionic Equation

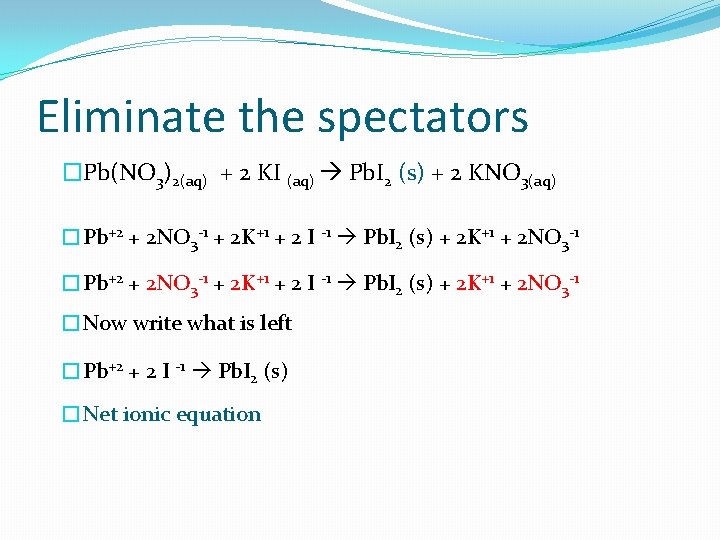
Eliminate the spectators �Pb(NO 3)2(aq) + 2 KI (aq) Pb. I 2 (s) + 2 KNO 3(aq) �Pb+2 + 2 NO 3 -1 + 2 K+1 + 2 I -1 Pb. I 2 (s) + 2 K+1 + 2 NO 3 -1 �Now write what is left �Pb+2 + 2 I -1 Pb. I 2 (s) �Net ionic equation