Renal Cell Carcinoma Nursing Considerations With the Use















- Slides: 50

Renal Cell Carcinoma: Nursing Considerations With the Use of Targeted Therapy Nancy Moldawer, RN, MSN Clinical Research Operations Manager Division of Medical Oncology and Therapeutic Research City of Hope Duarte, California

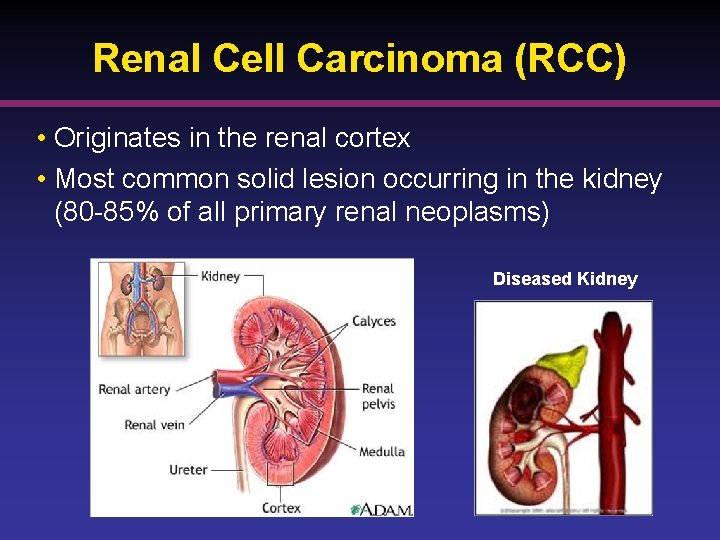
Renal Cell Carcinoma (RCC) • Originates in the renal cortex • Most common solid lesion occurring in the kidney (80 -85% of all primary renal neoplasms) Diseased Kidney

RCC Statistics • US estimates for 20071 § 51, 190 individuals diagnosed with cancer of the kidney and renal pelvis § 12, 890 individuals died from cancer of the kidney and renal pelvis • 3 rd most common genitourinary cancer after prostate cancer and bladder cancer 2 • Median age at diagnosis: 65 years (2000 -2004)1 • Median age at death: 71 years (2000 -2004)1 1. National Cancer Institute. SEER cancer statistics fact sheet: cancer of the kidney and renal pelvis. Accessed 2008. 2. Jemal A et al. CA Cancer J Clin. 2007; 57: 43.

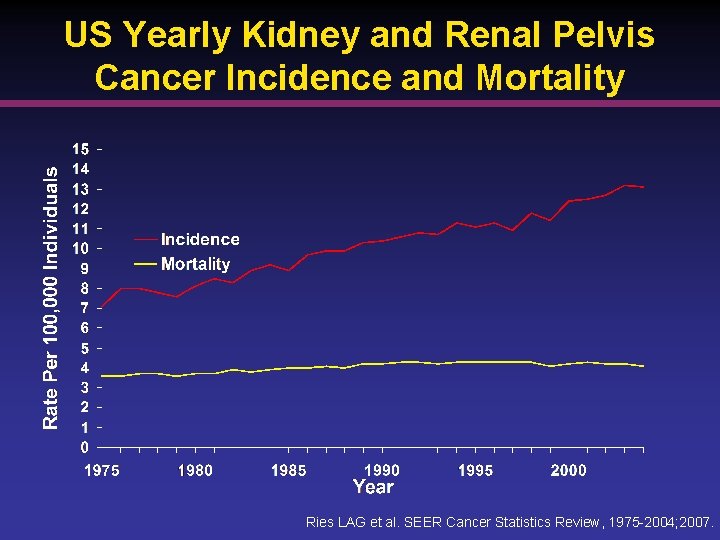
RCC Statistics • An estimated 240, 266 US individuals with a history of kidney and renal pelvis cancer were alive in 20041 • 5 -year survival has improved 2 § 50. 9% 1975 1977 § 65. 7% 1996 2003 1. National Cancer Institute. SEER cancer statistics fact sheet: cancer of the kidney and renal pelvis. Accessed 2008. 2. Ries LAG, et al. SEER Cancer Statistics Review. 2007; 1975 -2004.

US Yearly Kidney and Renal Pelvis Cancer Incidence and Mortality Ries LAG et al. SEER Cancer Statistics Review, 1975 -2004; 2007.

Etiology of RCC • Environmental and clinical risk factors § Smoking 1, 2 § Obesity 1, 3, 4 § Acquired cystic disease of the kidney (usually in association with dialysis)5, 6 § Analgesic abuse nephropathy 7, 8 § Occupational exposure to toxic compounds 9 -11 § Genetic predisposition 12 1. Setiawan VW et al. Am J Epidemiol. 2007; 166: 932. 2. Hunt JD et al. Int J Cancer. 2005; 114: 101. 3. Pischon T et al. J Natl Cancer Inst. 2006; 98: 920. 4. Chow WH et al. N Engl J Med. 2000; 343: 1305. 5. Brennan JF et al. Br J Urol. 1991; 67: 342. 6. Truong LD et al. Am J Kidney Dis. 1995; 26: 1. 7. Chow WH et al. Int J Cancer. 1994; 59: 467. 8. Lornoy W et al. Lancet. 1986; 1: 1271. 9. Mandel JS et al. Cancer. 1995; 61: 601. 10. Mc. Laughlin JK, Blot WJ. Int Arch Occup Environ Health. 1997; 70: 222. 11. Brauch H et al. Toxicol Lett. 2004; 151: 301. 12. Zbar B et al. J Urol. 2007; 177: 461.

Symptoms • Many patients with RCC are asymptomatic and have nonpalpable renal masses until late in natural disease course 1, 2 • Common local symptoms § Hematuria § Ipsilateral flank or abdominal pain § Palpable mass 1. Lee CT et al. Urol Oncol. 2002; 7: 135. 2. Patard JJ et al. Eur Urol. 2003; 44: 226.

Symptoms • Common systemic symptoms § Paraneoplastic disorders – Hypertension – Cachexia – Weight loss – Pyrexia – Neuromyopathy – Amyloidosis – Elevated erythrocyte sedimentation rate – Anemia – Abnormal liver function – Hypercalcemia – Polycythemia § Pain or mass related to metastatic disease

Physical Examination • Plays a limited role in diagnosing RCC • May be valuable in situations where there is § § A palpable abdominal mass A palpable cervical lymphadenopathy Non-reducing varicocele Bilateral lower extremity edema suggestive of venous involvement • Any of the above findings warrants radiologic examination Ljungberg B et al. Eur Urol. 2007; 51: 1502.

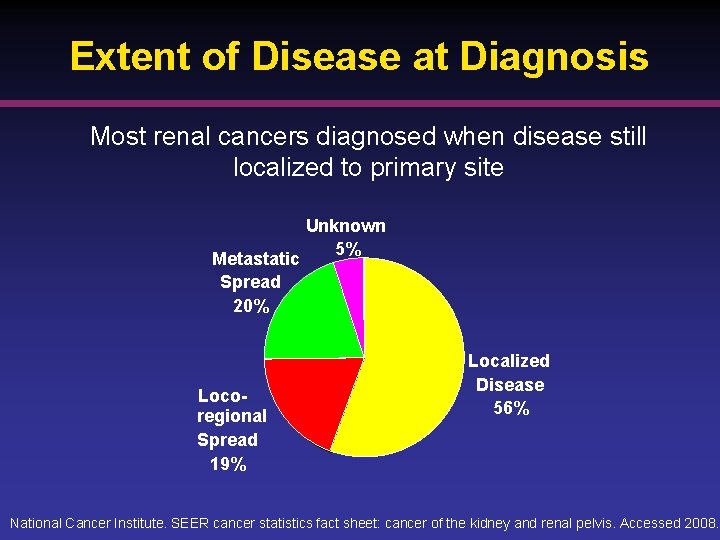
Extent of Disease at Diagnosis Most renal cancers diagnosed when disease still localized to primary site Metastatic Spread 20% Locoregional Spread 19% Unknown 5% Localized Disease 56% National Cancer Institute. SEER cancer statistics fact sheet: cancer of the kidney and renal pelvis. Accessed 2008.

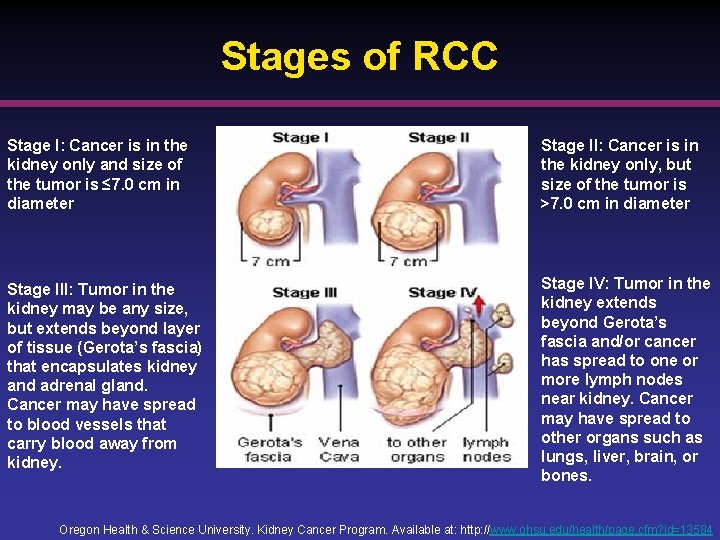
Stages of RCC Stage I: Cancer is in the kidney only and size of the tumor is ≤ 7. 0 cm in diameter Stage II: Cancer is in the kidney only, but size of the tumor is >7. 0 cm in diameter Stage III: Tumor in the kidney may be any size, but extends beyond layer of tissue (Gerota’s fascia) that encapsulates kidney and adrenal gland. Cancer may have spread to blood vessels that carry blood away from kidney. Stage IV: Tumor in the kidney extends beyond Gerota’s fascia and/or cancer has spread to one or more lymph nodes near kidney. Cancer may have spread to other organs such as lungs, liver, brain, or bones. Oregon Health & Science University. Kidney Cancer Program. Available at: http: //www. ohsu. edu/health/page. cfm? id=13584

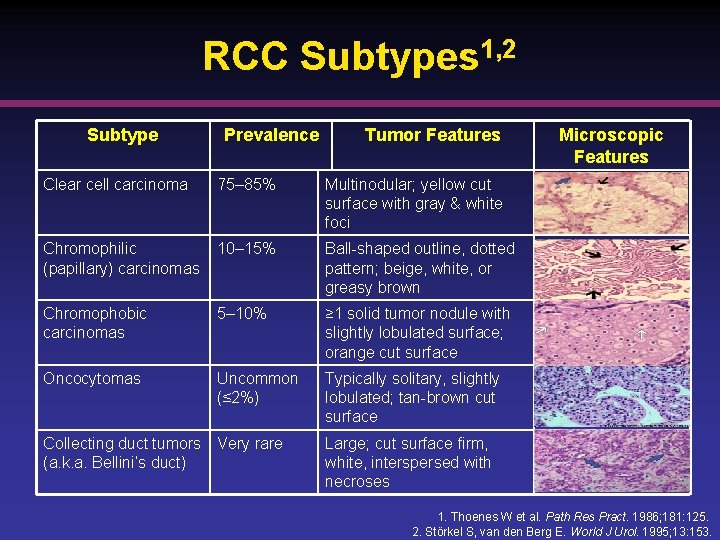
RCC Subtypes 1, 2 Subtype Prevalence Tumor Features Clear cell carcinoma 75– 85% Multinodular; yellow cut surface with gray & white foci Chromophilic (papillary) carcinomas 10– 15% Ball-shaped outline, dotted pattern; beige, white, or greasy brown Chromophobic carcinomas 5– 10% ≥ 1 solid tumor nodule with slightly lobulated surface; orange cut surface Oncocytomas Uncommon (≤ 2%) Typically solitary, slightly lobulated; tan-brown cut surface Collecting duct tumors Very rare (a. k. a. Bellini’s duct) Microscopic Features Large; cut surface firm, white, interspersed with necroses 1. Thoenes W et al. Path Res Pract. 1986; 181: 125. 2. Störkel S, van den Berg E. World J Urol. 1995; 13: 153.

Prognostic Clinical Factors • Several clinical factors associated with poor survival in patients with RCC § Poor performance status 1, 2 § Presence of RCC symptoms and/or paraneoplastic syndrome 1 -6 – Anemia, hypercalcemia, hepatopathy, thrombocytosis, fever, weight loss § Obesity 7 1. Zisman A et al. J Clin Oncol. 2001; 19: 1649. 2. Motzer RJ et al. J Clin Oncol. 1999; 17: 2530. 3. Suppiah R et al. Cancer. 2006; 107: 1793. 4. Bensalah K et al. J Urol 2006; 175: 859. 5. Fahn HJ et al. J Urol. 1991; 145: 248. 6. Patard JJ et al. J Urol. 2004; 172: 2167. 7. Calle EE et al. N Engl J Med. 2003; 348: 1625.

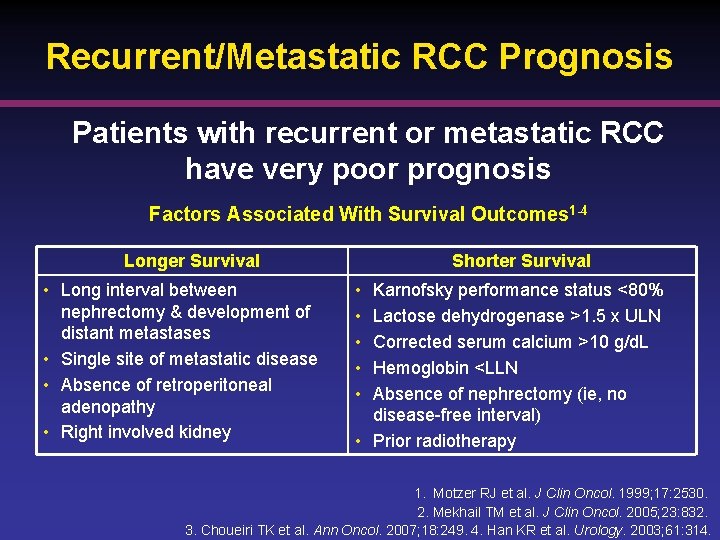
Recurrent/Metastatic RCC Prognosis Patients with recurrent or metastatic RCC have very poor prognosis Factors Associated With Survival Outcomes 1 -4 Longer Survival • Long interval between nephrectomy & development of distant metastases • Single site of metastatic disease • Absence of retroperitoneal adenopathy • Right involved kidney Shorter Survival • • • Karnofsky performance status <80% Lactose dehydrogenase >1. 5 x ULN Corrected serum calcium >10 g/d. L Hemoglobin <LLN Absence of nephrectomy (ie, no disease-free interval) • Prior radiotherapy 1. Motzer RJ et al. J Clin Oncol. 1999; 17: 2530. 2. Mekhail TM et al. J Clin Oncol. 2005; 23: 832. 3. Choueiri TK et al. Ann Oncol. 2007; 18: 249. 4. Han KR et al. Urology. 2003; 61: 314.

Advanced RCC • Treatment options other than surgery § Radiotherapy § Not an effective option § Chemotherapy – Not an effective option § Immunotherapy – Limited/some benefit § Targeted therapy – Clinical benefit; active area of research and further refinement 1. National Cancer Institute. SEER cancer statistics fact sheet: cancer of the kidney and renal pelvis. Accessed 2008. 2. Janzen N et al. Urol Clin North Am. 2003; 30: 843.

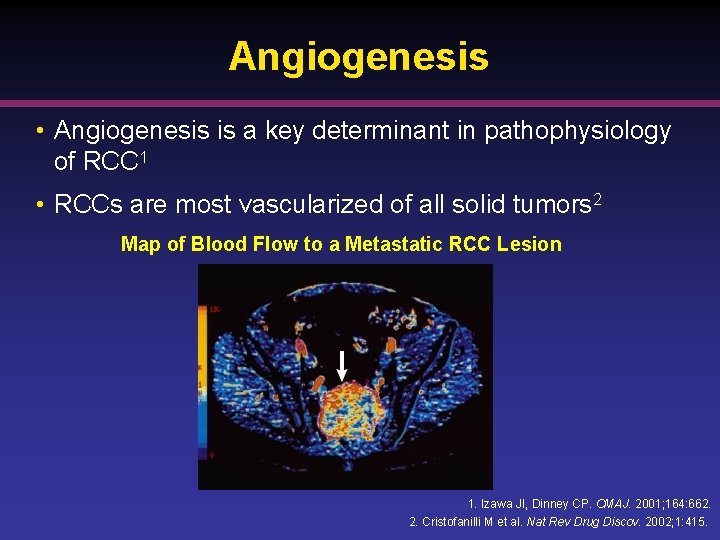
Angiogenesis • Angiogenesis is a key determinant in pathophysiology of RCC 1 • RCCs are most vascularized of all solid tumors 2 Map of Blood Flow to a Metastatic RCC Lesion 1. Izawa JI, Dinney CP. CMAJ. 2001; 164: 662. 2. Cristofanilli M et al. Nat Rev Drug Discov. 2002; 1: 415.

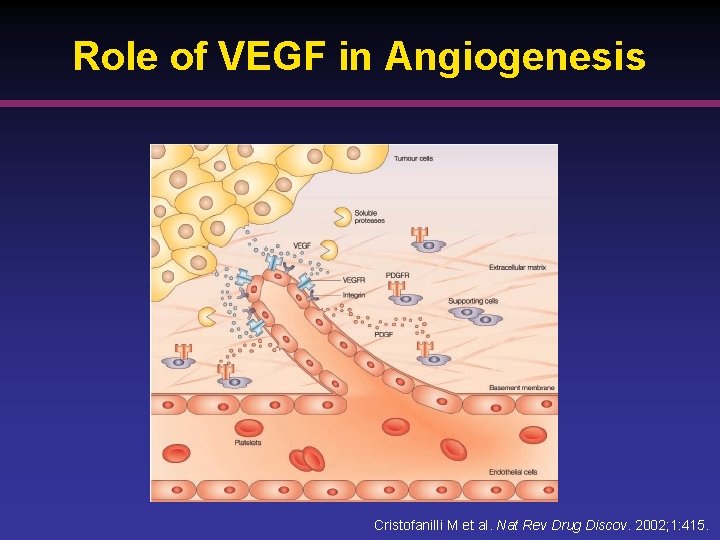
Role of VEGF in Angiogenesis Cristofanilli M et al. Nat Rev Drug Discov. 2002; 1: 415.

Growth Factors • Vascular endothelial growth factor (VEGF) key growth factor involved in angiogenesis 1, 2 § VEGF m. RNA expression correlates with vascularization § VEGF is overexpressed in most clear-cell RCCs • Platelet-derived growth factor (PDGF) and epidermal growth factor (EGF) play role in angiogenesis and oncogenesis 1. Cristofanilli M et al. Nat Rev Drug Discov. 2002; 1: 415. 2. De Mulder PH. Ann Oncol. 2007; 18: ix 98.

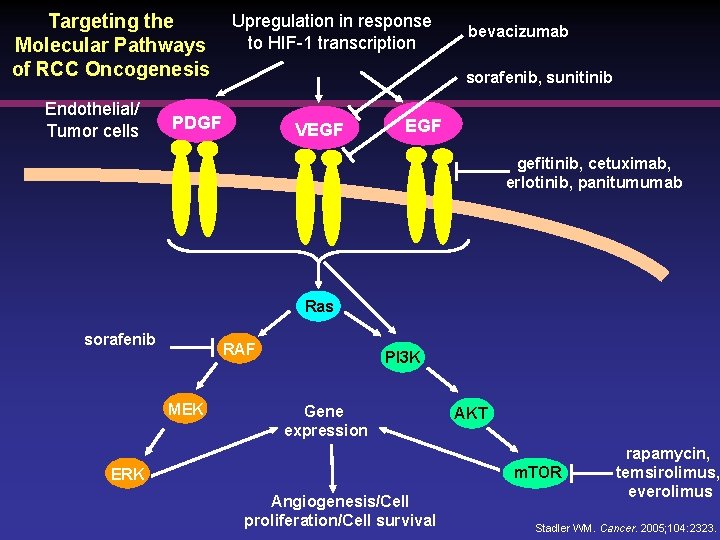
Targeting the Molecular Pathways of RCC Oncogenesis Endothelial/ Tumor cells Upregulation in response to HIF-1 transcription bevacizumab sorafenib, sunitinib PDGF VEGF gefitinib, cetuximab, erlotinib, panitumumab Ras sorafenib RAF MEK PI 3 K Gene expression AKT m. TOR ERK Angiogenesis/Cell proliferation/Cell survival rapamycin, temsirolimus, everolimus Stadler WM. Cancer. 2005; 104: 2323.

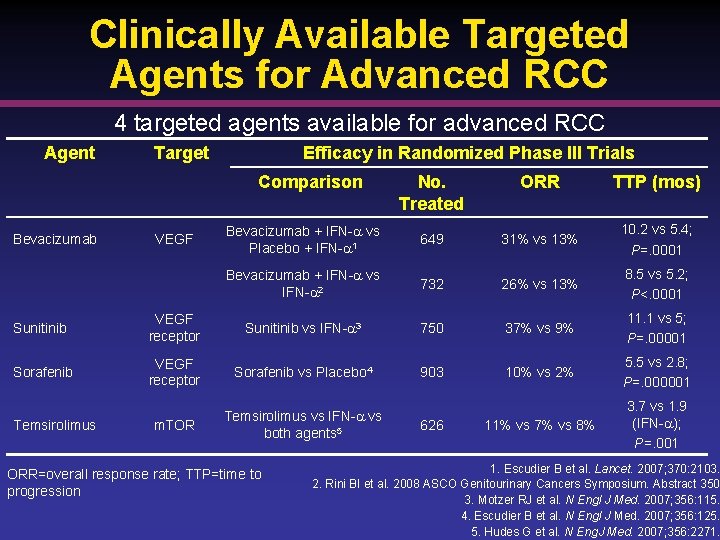
Clinically Available Targeted Agents for Advanced RCC 4 targeted agents available for advanced RCC Agent Target Efficacy in Randomized Phase III Trials Comparison Bevacizumab VEGF No. Treated ORR TTP (mos) Bevacizumab + IFN- vs Placebo + IFN- 1 649 31% vs 13% 10. 2 vs 5. 4; P=. 0001 Bevacizumab + IFN- vs IFN- 2 732 26% vs 13% 8. 5 vs 5. 2; P<. 0001 Sunitinib VEGF receptor Sunitinib vs IFN- 3 750 37% vs 9% 11. 1 vs 5; P=. 00001 Sorafenib VEGF receptor Sorafenib vs Placebo 4 903 10% vs 2% 5. 5 vs 2. 8; P=. 000001 m. TOR Temsirolimus vs IFN- vs both agents 5 11% vs 7% vs 8% 3. 7 vs 1. 9 (IFN- ); P=. 001 Temsirolimus ORR=overall response rate; TTP=time to progression 626 1. Escudier B et al. Lancet. 2007; 370: 2103. 2. Rini BI et al. 2008 ASCO Genitourinary Cancers Symposium. Abstract 350 3. Motzer RJ et al. N Engl J Med. 2007; 356: 115. 4. Escudier B et al. N Engl J Med. 2007; 356: 125. 5. Hudes G et al. N Eng. J Med. 2007; 356: 2271.

Integrating the Oncology Nurse Into the New Paradigm of Targeted Therapy • The new therapeutic paradigm of moleculartargeted therapy presents new challenges for oncology nurses § Induces tumor stabilization vs complete responses § Controls disease vs curing disease § Unique side-effect profiles § As the landscape of RCC treatment continues to evolve, the nurse remains on the forefront of drug discovery, administration, and adverse event monitoring Moldawer N, Wood LS. Kidney Cancer J. 2006; 4: 25 -32.

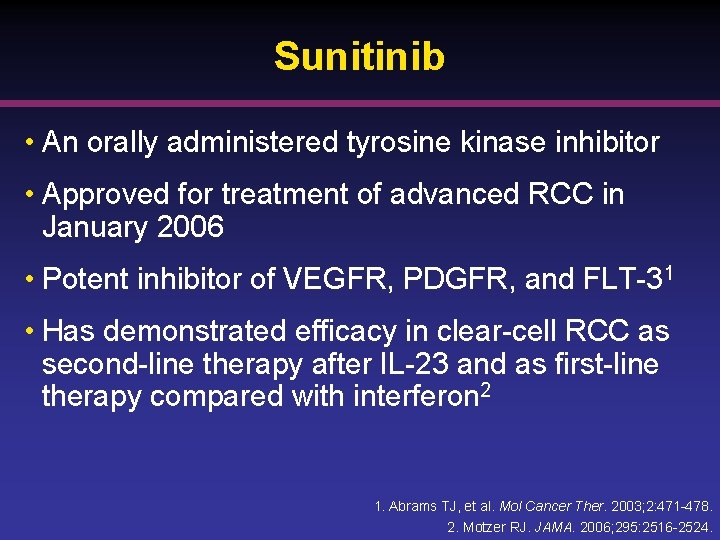
Sunitinib • An orally administered tyrosine kinase inhibitor • Approved for treatment of advanced RCC in January 2006 • Potent inhibitor of VEGFR, PDGFR, and FLT-31 • Has demonstrated efficacy in clear-cell RCC as second-line therapy after IL-23 and as first-line therapy compared with interferon 2 1. Abrams TJ, et al. Mol Cancer Ther. 2003; 2: 471 -478. 2. Motzer RJ. JAMA. 2006; 295: 2516 -2524.

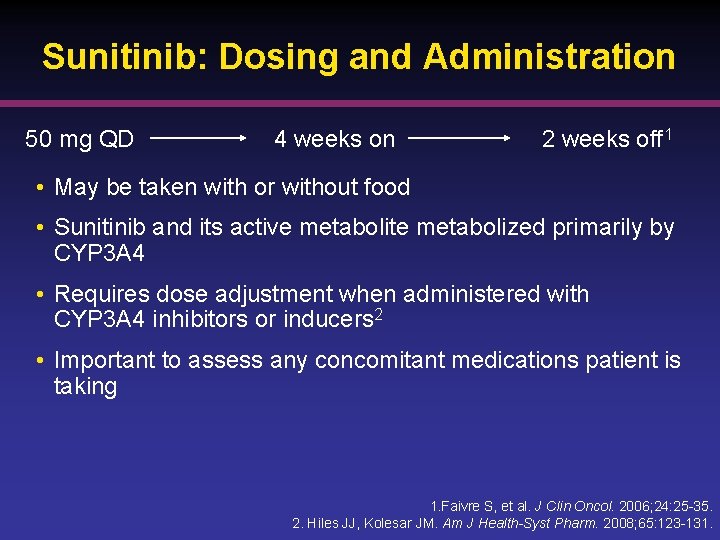
Sunitinib: Dosing and Administration 50 mg QD 4 weeks on 2 weeks off 1 • May be taken with or without food • Sunitinib and its active metabolite metabolized primarily by CYP 3 A 4 • Requires dose adjustment when administered with CYP 3 A 4 inhibitors or inducers 2 • Important to assess any concomitant medications patient is taking 1. Faivre S, et al. J Clin Oncol. 2006; 24: 25 -35. 2. Hiles JJ, Kolesar JM. Am J Health-Syst Pharm. 2008; 65: 123 -131.

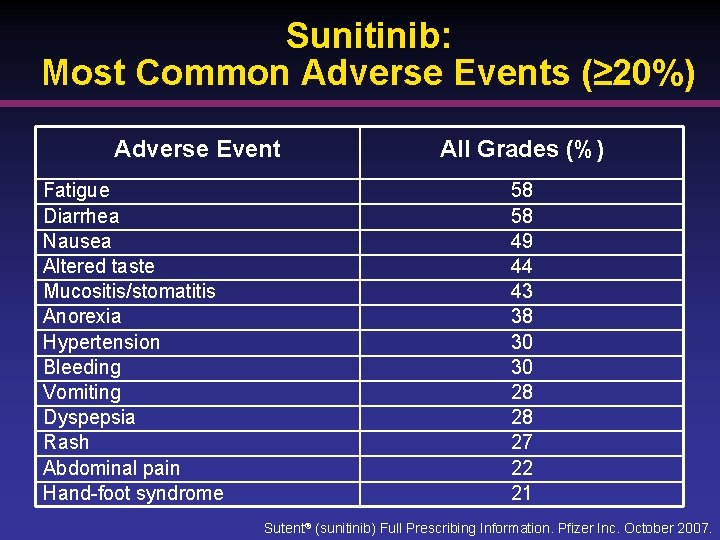
Sunitinib: Most Common Adverse Events (≥ 20%) Adverse Event Fatigue Diarrhea Nausea Altered taste Mucositis/stomatitis Anorexia Hypertension Bleeding Vomiting Dyspepsia Rash Abdominal pain Hand-foot syndrome All Grades (%) 58 58 49 44 43 38 30 30 28 28 27 22 21 Sutent® (sunitinib) Full Prescribing Information. Pfizer Inc. October 2007.

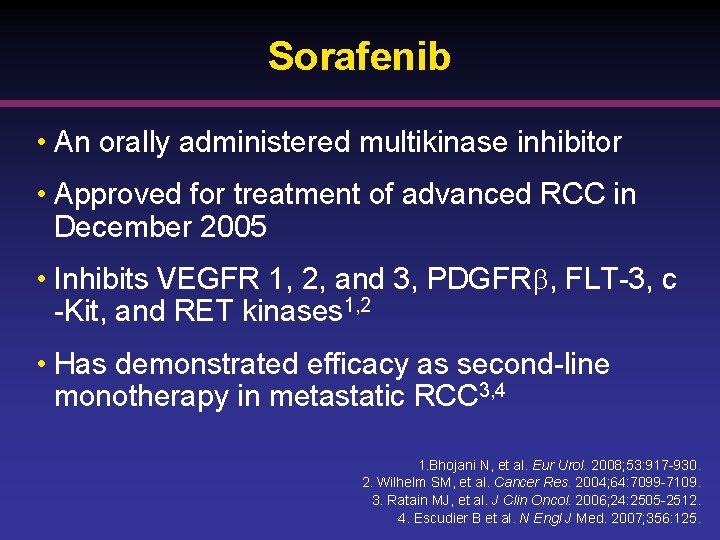
Sorafenib • An orally administered multikinase inhibitor • Approved for treatment of advanced RCC in December 2005 • Inhibits VEGFR 1, 2, and 3, PDGFR , FLT-3, c -Kit, and RET kinases 1, 2 • Has demonstrated efficacy as second-line monotherapy in metastatic RCC 3, 4 1. Bhojani N, et al. Eur Urol. 2008; 53: 917 -930. 2. Wilhelm SM, et al. Cancer Res. 2004; 64: 7099 -7109. 3. Ratain MJ, et al. J Clin Oncol. 2006; 24: 2505 -2512. 4. Escudier B et al. N Engl J Med. 2007; 356: 125.

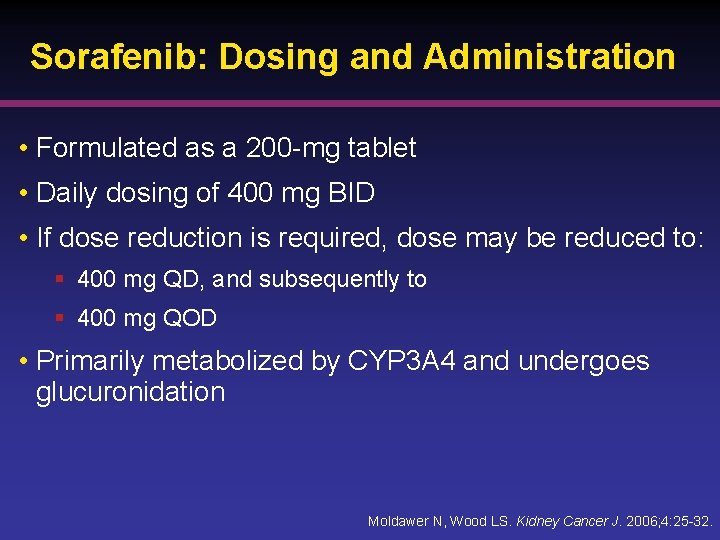
Sorafenib: Dosing and Administration • Formulated as a 200 -mg tablet • Daily dosing of 400 mg BID • If dose reduction is required, dose may be reduced to: § 400 mg QD, and subsequently to § 400 mg QOD • Primarily metabolized by CYP 3 A 4 and undergoes glucuronidation Moldawer N, Wood LS. Kidney Cancer J. 2006; 4: 25 -32.

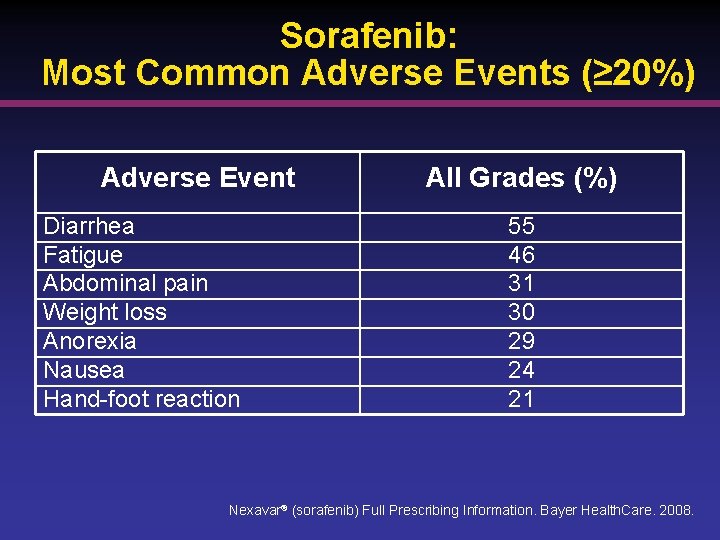
Sorafenib: Most Common Adverse Events (≥ 20%) Adverse Event Diarrhea Fatigue Abdominal pain Weight loss Anorexia Nausea Hand-foot reaction All Grades (%) 55 46 31 30 29 24 21 Nexavar® (sorafenib) Full Prescribing Information. Bayer Health. Care. 2008.

Sunitinib and Sorafenib Adverse Events: Nursing Implications 1, 2 • Diarrhea § Treat initially with diet modification (low residue) and loperamide § If loperamide insufficient, give diphenoxylate HCl with atropine § Additional options include tincture of opium, Culturelle (oral probiotic), and Dannon yogurt containing bifidobacterium • Fatigue § Adjust activities to allow for rest periods and maximize fluid and caloric intake 1. Wood LS. Oncology Nurs News. 2007; 3: 19 -20. 2. Wood LS. Oncology Nurs News. 2007; 4: 37 -38.

Sunitinib and Sorafenib Adverse Events: Nursing Implications 1, 2 (cont. ) • Functional or clinical mucositis Dietary modifications § Avoidance of carbonated beverages and spicy foods § Eating foods at room temperature Medications § Topical lidocaine or Xylocaine § BMX Solution (Benadryl/Mylanta, Xylocaine) § Rincinol (OTC topical solution containing aloe vera) § Nystatin suspension or clotrimazole troches for clinical mucositis 1. Wood LS. Oncology Nurs News. 2007; 3: 19 -20. 2. Wood LS. Oncology Nurs News. 2007; 4: 37 -38.

Sunitinib and Sorafenib Adverse Events: Nursing Implications 1, 2 (cont. ) • Taste changes and anorexia § Maximize caloric intake § Encourage 6 small meals per day § Use of flavorings and gravy to enhance food taste • Hand-foot reaction § Liberal use of emollients § Avoid activities that cause pressure, abrasion, or irritation to hands and feet § Application of Udderly Smooth lotion BID Other options include: § Bag Balm § Aveeno Skin Relief Moisturizing Cream § Aveeno Intense Relief Foot Cream § Kerasal ointment § Keralec cream § Zim’s Crack Crème § Biafine Topical Emulsion 1. Wood LS. Oncology Nurs News. 2007; 3: 19 -20. 2. Wood LS. Oncology Nurs News. 2007; 4: 37 -38.

Bevacizumab • Monoclonal antibody to VEGF active in multiple tumor types • First biological antiangiogenic agent approved by US FDA • Approved for use in colorectal, non-small cell lung, and metastatic breast cancers 1 • Phase 3 studies are evaluating bevacizumab in a variety of solid tumor types 2 1. Avastin® (bevacizumab) Full Prescribing Information. Genentech, Inc. March 2008. 2. National Cancer Institute website. http: //www. cancer. gov/clinicaltrials/search.

Bevacizumab: Dosing and Administration • Usual dosage of bevacizumab for treatment of colorectal cancer is 5 mg/kg IV every 2 weeks • Dosage in investigational trials of RCC has generally been 10 mg/kg IV every 2 weeks 1, 2 • Can be associated with hypersensitivity reactions 1. Hainsworth JD, et al. J Clin Oncol. 2005; 23: 7889 -7996. 2. Bukowski RM, et al. J Clin Oncol. 2007; 25: 4536 -4541.

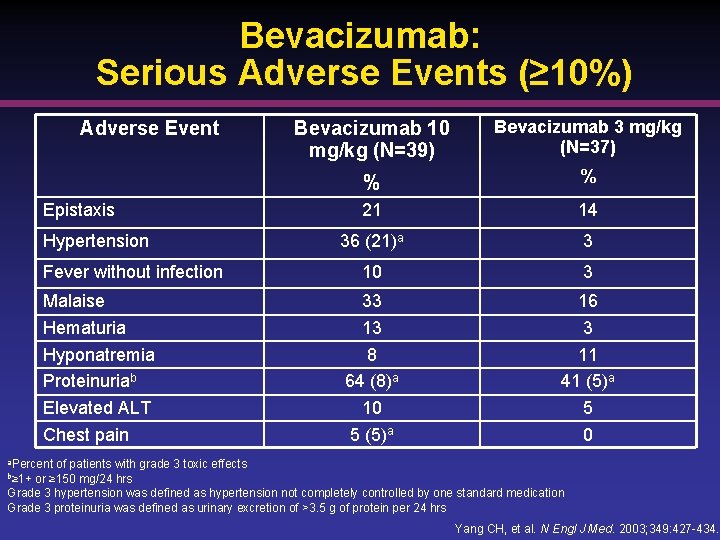
Bevacizumab: Serious Adverse Events (≥ 10%) Adverse Event Epistaxis Hypertension Fever without infection Malaise Hematuria Hyponatremia Proteinuriab Elevated ALT Chest pain Bevacizumab 10 mg/kg (N=39) Bevacizumab 3 mg/kg (N=37) % % 21 14 36 (21)a 3 10 3 33 13 8 64 (8)a 10 5 (5)a 16 3 11 41 (5)a 5 0 a. Percent of patients with grade 3 toxic effects or ≥ 150 mg/24 hrs Grade 3 hypertension was defined as hypertension not completely controlled by one standard medication Grade 3 proteinuria was defined as urinary excretion of >3. 5 g of protein per 24 hrs b≥ 1+ Yang CH, et al. N Engl J Med. 2003; 349: 427 -434.

Bevacizumab Adverse Events: Nursing Implications • Bleeding § Obtain patient history of unusual bleeding or clotting, GI perforation, and use of anticoagulants § Avoid anticoagulant therapy if possible, especially concomitant use of bevacizumab with warfarin and 5 -FU § Educate patient about signs of bleeding (ie, epistaxis, bleeding gums during tooth brushing, red or black, tarry stools, vomiting blood) • Thrombosis § Educate patient about signs of thrombosis that include – Sudden chest pain – Difficulty breathing Ignoffo RJ. Am J Health-Syst Pharm. 2004; 61(Suppl 5): 21 -26.

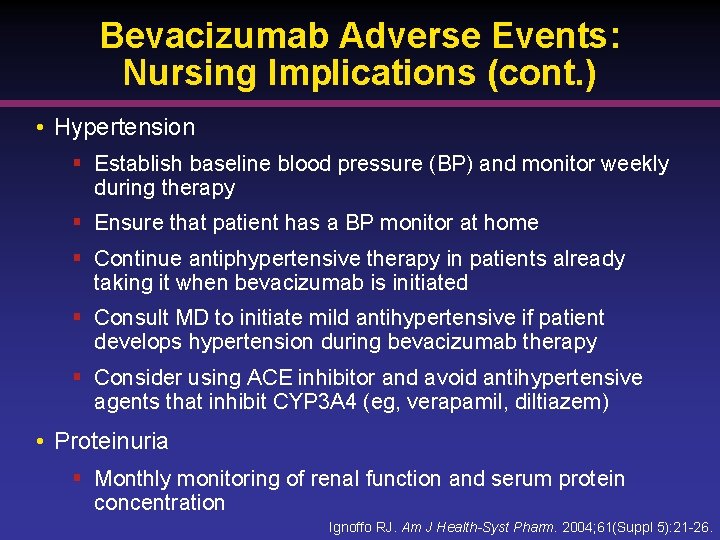
Bevacizumab Adverse Events: Nursing Implications (cont. ) • Hypertension § Establish baseline blood pressure (BP) and monitor weekly during therapy § Ensure that patient has a BP monitor at home § Continue antiphypertensive therapy in patients already taking it when bevacizumab is initiated § Consult MD to initiate mild antihypertensive if patient develops hypertension during bevacizumab therapy § Consider using ACE inhibitor and avoid antihypertensive agents that inhibit CYP 3 A 4 (eg, verapamil, diltiazem) • Proteinuria § Monthly monitoring of renal function and serum protein concentration Ignoffo RJ. Am J Health-Syst Pharm. 2004; 61(Suppl 5): 21 -26.

Temsirolimus • An inhibitor of mammalian target of rapamycin (m. TOR) kinase, a component of intracellular signaling pathways 1 • Binds to an abundant intracellular protein FKBP 12, forming a complex that inhibits m. TOR 2, 3 • First m. TOR inhibitor approved for treatment of advanced RCC • Approved by FDA on May 30, 2007 1. Schmelzle T, Hall MN. Cell. 2000; 103: 253 -262. 2. Skotnicki JS, et al. Clin Cancer Res. 2001; 7(Suppl): 3749 -3750. 3. Harding MW. Clin Cancer Res. 2003; 9: 2882 -2886.

Temsirolimus: Dosing and Administration • Approved dose for advanced RCC is 25 mg IV weekly over a 30 - to 60 -minute period • Patients should receive prophylactic diphenhydramine 25 50 mg IV prior to the start of each dose • If patient develops a hypersensitivity reaction during infusion: § § Stop infusion Observe patient at least 30 60 minutes Notify MD Treatment may be resumed at discretion of MD with administration of an H 1 -receptor antagonist (eg, diphenhydramine), if not previously administered, and/or an H 2 -receptor antagonist (eg, famotidine 20 mg IV or ranitidine 50 mg IV) 30 minutes before restarting temsirolimus § Extend infusion time to 60 minutes Torisel™ (temsirolimus) Full Prescribing Information. Wyeth Pharmaceuticals. May 2007.

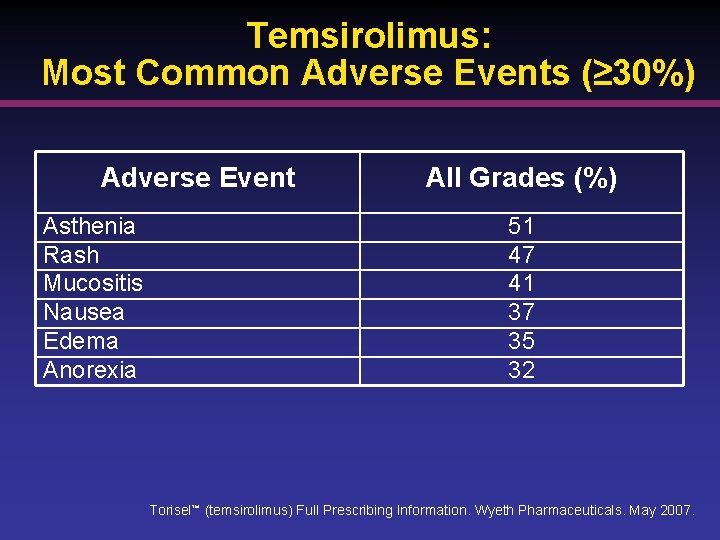
Temsirolimus: Most Common Adverse Events (≥ 30%) Adverse Event Asthenia Rash Mucositis Nausea Edema Anorexia All Grades (%) 51 47 41 37 35 32 Torisel™ (temsirolimus) Full Prescribing Information. Wyeth Pharmaceuticals. May 2007.

Temsirolimus Adverse Events: Nursing Implications • Rash § Observe for acne-like rash § Consider antihistamines for itching § Counsel patient to use skin emollients, avoid agents that cause skin drying effects, and avoid sun exposure • Anemia § Monitor hemoglobin and hematocrit regularly during therapy • Anorexia § Maximize caloric intake

Temsirolimus Adverse Events: Nursing Implications (cont. ) • Hyperlipidemia § Monitor serum cholesterol and triglycerides prior to and during therapy • Hyperglycemia § Monitor serum glucose prior to and periodically during therapy • Infection § Monitor for sore throat, appearance of sputum, urine, and stool § Monitor vital signs regularly § Educate patient about recognizing signs of infection

Temsirolimus Adverse Events: Nursing Implications (cont. ) • Interaction with CYP 3 A 4 inhibitors § Avoid concomitant use of temsirolimus with: – – – Grapefruit juice Ketoconazole Itraconazole Clarithromycin Atazanavir Indinavir Nefazodone Nelfinavir Ritonavir Saquinavir Telithromycin Vorizonazole Torisel™ (temsirolimus) Full Prescribing Information. Wyeth Pharmaceuticals. May 2007.

Temsirolimus Adverse Events: Nursing Implications (cont. ) • Interaction with CYP 3 A 4 inducers § Avoid concomitant use of temsirolimus with: – – – Dexamethasone Phenytoin Carbamazepine Phenobarbital Rifampin Rifabutin Torisel™ (temsirolimus) Full Prescribing Information. Wyeth Pharmaceuticals. May 2007.

NCCN Guidelines: Explanation of Categories of Evidence • Category 1 § Recommendation based on high-level evidence (ie, high-powered randomized clinical trials or meta-analyses) § NCCN Guidelines Panel has reached uniform consensus that the recommendation is indicated • Category 2 A § Recommendation based on lower-level evidence § Lower-level evidence is interpreted broadly and may range from phase 2 to large cohort studies to case studies § In many instances, retrospective studies derived from clinical experience of treating large numbers of patients and Guidelines Panel members have first-hand knowledge of data National Comprehensive Cancer Network. Available at: http: //www. nccn. org.

NCCN Guidelines: Explanation of Categories of Evidence • Category 2 B § Recommendation based on lower-level evidence § There is nonuniform consensus that the recommendation should be made § In these instances, institutions take different approaches to the management of a particular clinical scenario • Category 3 § Including the recommendation has engendered a major disagreement among NCCN Guidelines Panel members § Level of evidence not pertinent in this category because experts can disagree about the significance of high-level trials National Comprehensive Cancer Network. Available at: http: //www. nccn. org.

NCCN Practice Guidelines in Oncology: Kidney Cancer FIRST-LINE THERAPY Predominant clear cell histology Relapse or Stage IV and medically or surgically unresectable a. Temsirolimus indicated for poor-prognosis patients, defined as those with ≥ 3 predictors of short survival b. Best supportive care can include palliative RT, metastasectomy or biphosphonates for bony metastasis Clinical trial or Sunitinib (category 1) or Temsirolimus for poor-prognosis patientsa (category 1) or Bevacizumab + IFN or High-dose IL-2 for selected patients or Sorafenib for selected patients and Best supportive careb NCCN Clinical Practice Guidelines in Oncology. 2007; v. 1. 2008.

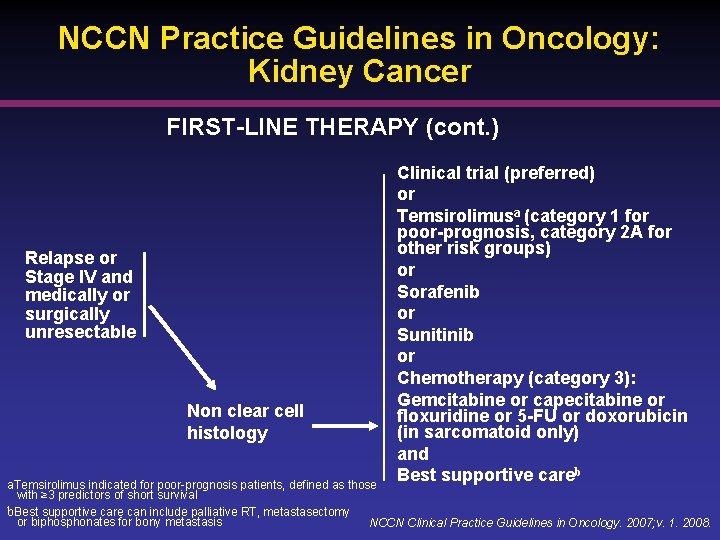
NCCN Practice Guidelines in Oncology: Kidney Cancer FIRST-LINE THERAPY (cont. ) Relapse or Stage IV and medically or surgically unresectable Non clear cell histology Clinical trial (preferred) or Temsirolimusa (category 1 for poor-prognosis, category 2 A for other risk groups) or Sorafenib or Sunitinib or Chemotherapy (category 3): Gemcitabine or capecitabine or floxuridine or 5 -FU or doxorubicin (in sarcomatoid only) and Best supportive careb a. Temsirolimus indicated for poor-prognosis patients, defined as those with ≥ 3 predictors of short survival b. Best supportive care can include palliative RT, metastasectomy or biphosphonates for bony metastasis NCCN Clinical Practice Guidelines in Oncology. 2007; v. 1. 2008.

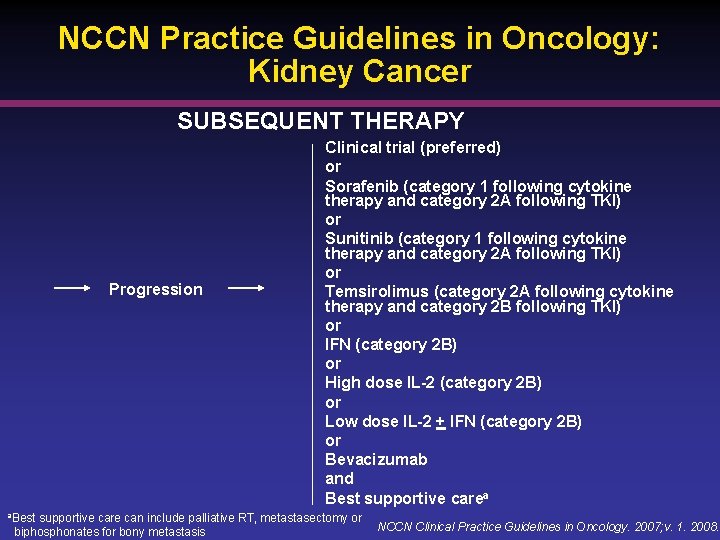
NCCN Practice Guidelines in Oncology: Kidney Cancer SUBSEQUENT THERAPY Progression a. Best Clinical trial (preferred) or Sorafenib (category 1 following cytokine therapy and category 2 A following TKI) or Sunitinib (category 1 following cytokine therapy and category 2 A following TKI) or Temsirolimus (category 2 A following cytokine therapy and category 2 B following TKI) or IFN (category 2 B) or High dose IL-2 (category 2 B) or Low dose IL-2 + IFN (category 2 B) or Bevacizumab and Best supportive carea supportive care can include palliative RT, metastasectomy or biphosphonates for bony metastasis NCCN Clinical Practice Guidelines in Oncology. 2007; v. 1. 2008.

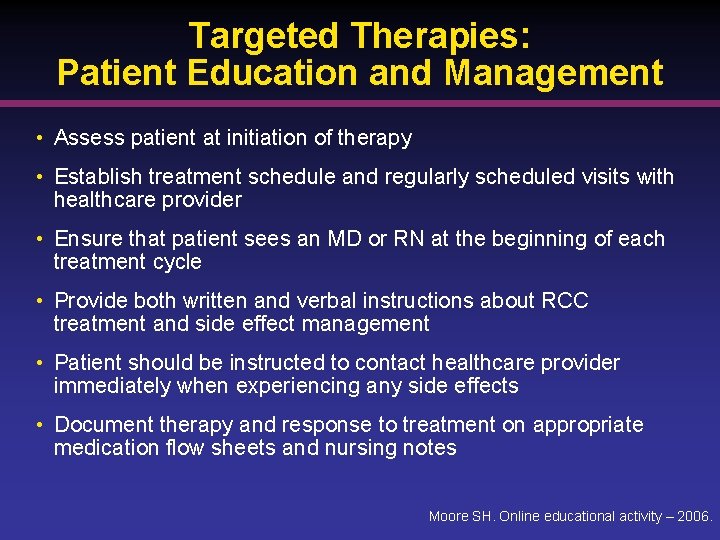
Targeted Therapies: Patient Education and Management • Assess patient at initiation of therapy • Establish treatment schedule and regularly scheduled visits with healthcare provider • Ensure that patient sees an MD or RN at the beginning of each treatment cycle • Provide both written and verbal instructions about RCC treatment and side effect management • Patient should be instructed to contact healthcare provider immediately when experiencing any side effects • Document therapy and response to treatment on appropriate medication flow sheets and nursing notes Moore SH. Online educational activity – 2006.

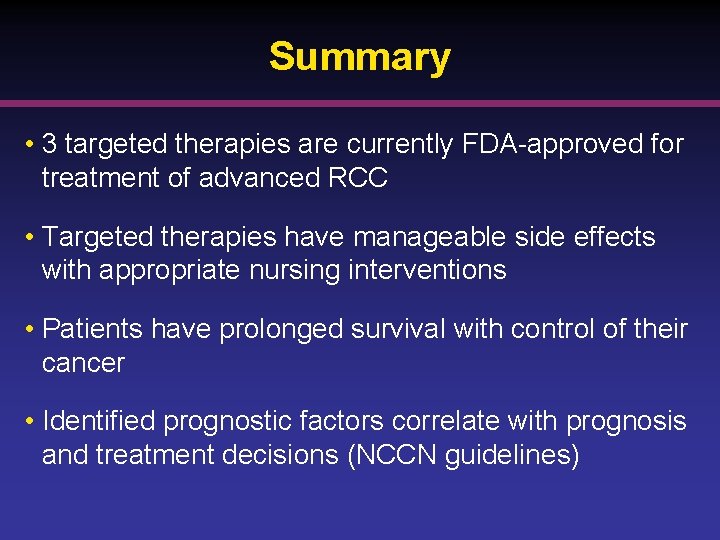
Summary • 3 targeted therapies are currently FDA-approved for treatment of advanced RCC • Targeted therapies have manageable side effects with appropriate nursing interventions • Patients have prolonged survival with control of their cancer • Identified prognostic factors correlate with prognosis and treatment decisions (NCCN guidelines)

Future Considerations • Do these targeted therapies have a role in the adjuvant setting? • Do combinations of these targeted therapies offer better clinical results or cause increased toxicity? • Patient preference: oral or IV therapy?