RELATIVE MASS AND ATOMIC MASS 1 In the





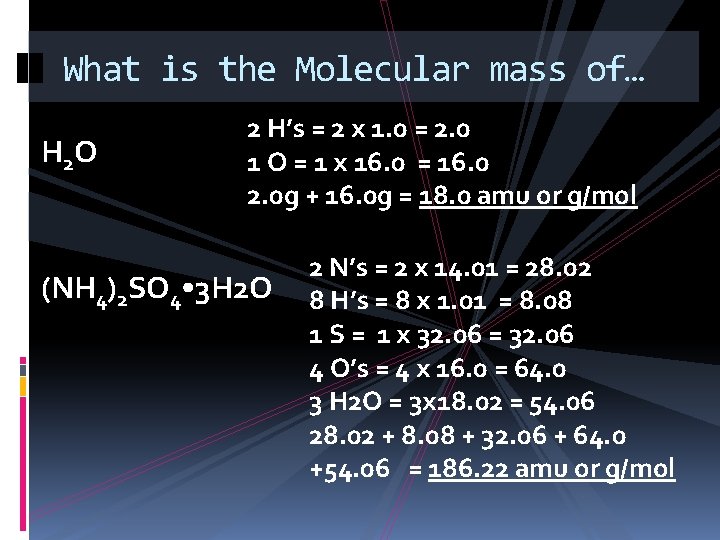
- Slides: 15

RELATIVE MASS AND ATOMIC MASS

1. In the late 1700’s, a French scientist named J. L. Gay-Lussac investigated how gaseous elements combined to form gaseous compounds. ü He found that elements always joined in simple whole number ratios by their volumes. ü Ie. H 2 O = 1 O to 2 H's

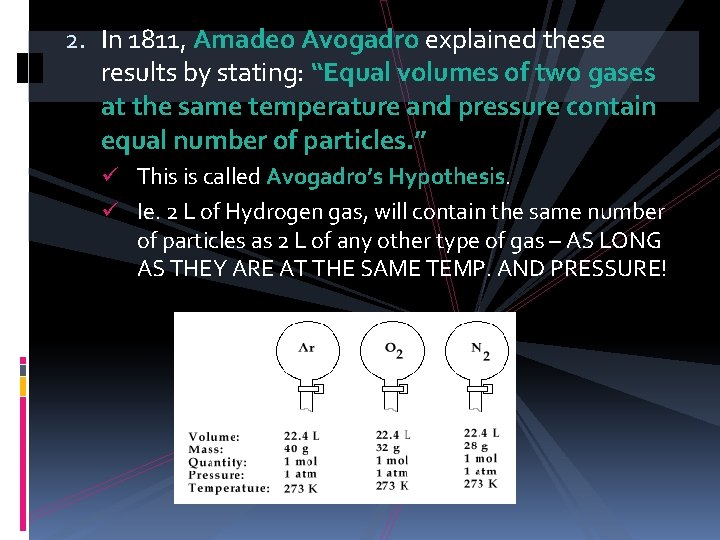
2. In 1811, Amadeo Avogadro explained these results by stating: “Equal volumes of two gases at the same temperature and pressure contain equal number of particles. ” ü This is called Avogadro’s Hypothesis. ü Ie. 2 L of Hydrogen gas, will contain the same number of particles as 2 L of any other type of gas – AS LONG AS THEY ARE AT THE SAME TEMP. AND PRESSURE!

In other words if 1 L of gas A reacts with 1 L of gas B, then there are exactly as many particles of A present as B. Therefore, 1 atom of A reacts with 1 atom of B to make the formula AB. If 2 L of gas A reacts with 1 L of gas B, then there will be twice as many atoms of A as there is B (2 atoms of A combine with 1 atom of B). The formula is A 2 B. Question: If 1 L of Nitrogen gas reacts with 3 L of Hydrogen gas, at the same temperature and pressure, what would the formula and name of the resulting compound be? Answer: NH 3 – Nitrogen Trihydride Question: If 500 ml of Iodine gas reacts with 2 L of Bromine gas, at the same temperature and pressure, what would the formula and name of the resulting compound be? Answer: IBr 4 – Iodine Tetrabromide

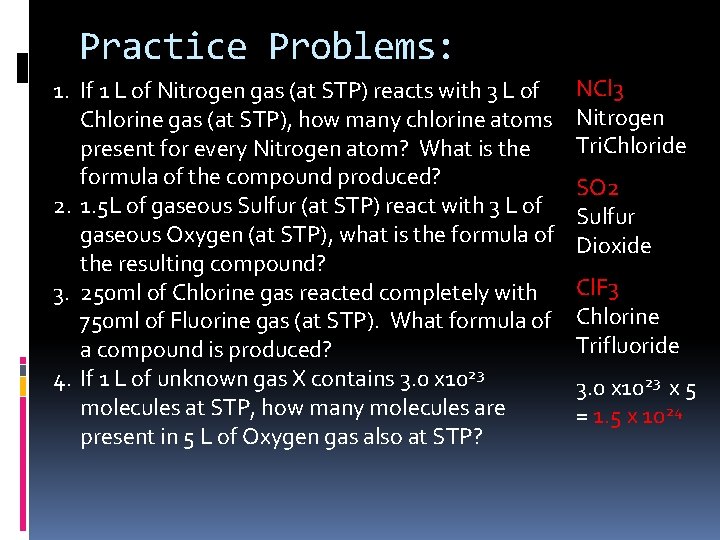
Practice Problems: 1. If 1 L of Nitrogen gas (at STP) reacts with 3 L of Chlorine gas (at STP), how many chlorine atoms present for every Nitrogen atom? What is the formula of the compound produced? 2. 1. 5 L of gaseous Sulfur (at STP) react with 3 L of gaseous Oxygen (at STP), what is the formula of the resulting compound? 3. 250 ml of Chlorine gas reacted completely with 750 ml of Fluorine gas (at STP). What formula of a compound is produced? 4. If 1 L of unknown gas X contains 3. 0 x 1023 molecules at STP, how many molecules are present in 5 L of Oxygen gas also at STP? NCl 3 Nitrogen Tri. Chloride SO 2 Sulfur Dioxide Cl. F 3 Chlorine Trifluoride 3. 0 x 1023 x 5 = 1. 5 x 1024

3. John Dalton, an English school teacher, took the work of Gay-Lussac and Avogadro’s step further in developing the idea of relative masses for the elements. ü Relative mass is a mathematical comparison between an object and an equal number of another object.

Since he didn’t know the actual mass of each atom of these gases, he assigned hydrogen a mass of 1. 00 as hydrogen was the lightest known element. 1. Therefore, chlorine had a relative mass of 35. 5. 2. Every known element was then compared to hydrogen.

ü Later, the mass of the elements were compared to oxygen… … do you know why? ? ? 1. More abundant 2. Less dangerous!

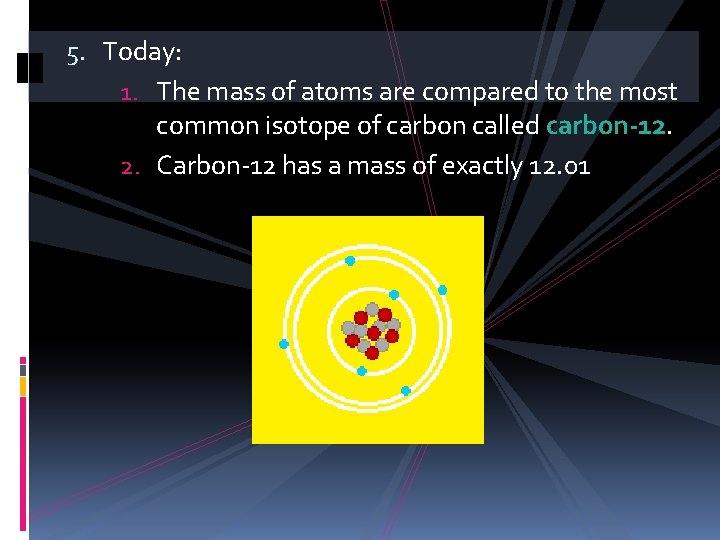
5. Today: 1. The mass of atoms are compared to the most common isotope of carbon called carbon-12. 2. Carbon-12 has a mass of exactly 12. 01

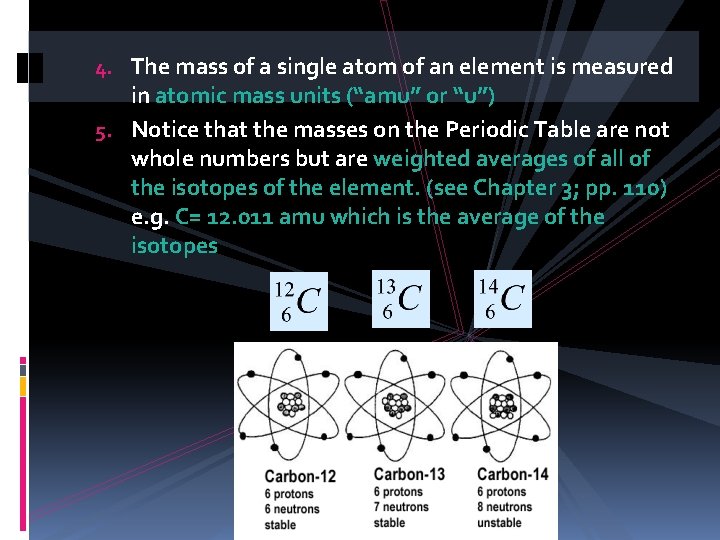
4. The mass of a single atom of an element is measured in atomic mass units (“amu” or “u”) 5. Notice that the masses on the Periodic Table are not whole numbers but are weighted averages of all of the isotopes of the element. (see Chapter 3; pp. 110) e. g. C= 12. 011 amu which is the average of the isotopes

Molecular Mass Cu(C 2 H 3 O 2)2 What elements and how many of each are present in this compound? 1 Cu, 4 C, 6 H, 4 O If we consider the mass of each element independently, the mass of the molecule is the sum of its elements!

So what is the Molecular mass of Cu(C 2 H 3 O 2)2 ? ? 1 Cu = 63. 55 amu 4 C = 4(12. 01 amu) = 48. 04 amu 6 H = 6(1. 01 amu) = 6. 06 amu 4 O = 4(16. 00 amu) = 64 amu So… 63. 55 + 48. 04 + 6. 06 + 64 = Molecular mass = 181. 65 amu!

What would be the Molecular mass of Na. Cl? 1 Na = 22. 99 amu 1 Cl = 35. 45 amu So… 22. 99 amu + 35. 45 amu 58. 49 amu Therefore, the molecular mass of Na. Cl is 58. 49 amu
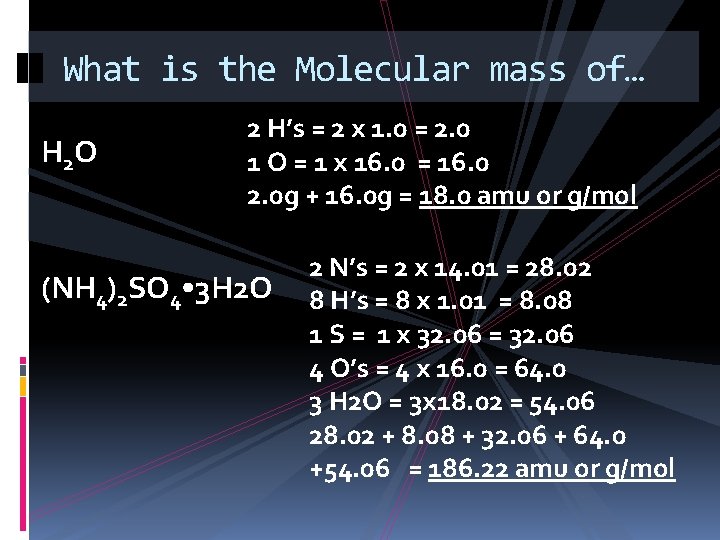
What is the Molecular mass of… H 2 O 2 H’s = 2 x 1. 0 = 2. 0 1 O = 1 x 16. 0 = 16. 0 2. 0 g + 16. 0 g = 18. 0 amu or g/mol (NH 4)2 SO 4 • 3 H 2 O 2 N’s = 2 x 14. 01 = 28. 02 8 H’s = 8 x 1. 01 = 8. 08 1 S = 1 x 32. 06 = 32. 06 4 O’s = 4 x 16. 0 = 64. 0 3 H 2 O = 3 x 18. 02 = 54. 06 28. 02 + 8. 08 + 32. 06 + 64. 0 +54. 06 = 186. 22 amu or g/mol

Let’s try figuring out Molecular Mass! Hebden Pg. 80 # 6 a, c, e, g, i, l, n, p # 7 a, c