Relative energy levels s of electrons in gaseous






- Slides: 10

Relative energy levels s of electrons in gaseous atoms of the first twenty elements Work through this p d f Electronic Structure tutorial in sequence, or go directly to the section required using the links below. Use the ‘home’ button (to right) to return to this screen. Energy levels within atoms: sub-levels and orbitals Filling energy levels with electrons Electronic configuration notation Increasing energy Ionisation 1 s Credits

Relative energy levels of electrons in gaseous atoms of the first twenty elements s Increasing energy 1 s p d f

Relative energy levels of electrons in gaseous atoms of the first twenty elements s 2 p 2 s Increasing energy 1 s p d f

Relative energy levels of electrons in gaseous atoms of the first twenty elements s 3 d 3 p 3 s 2 p 2 s Increasing energy 1 s p d f

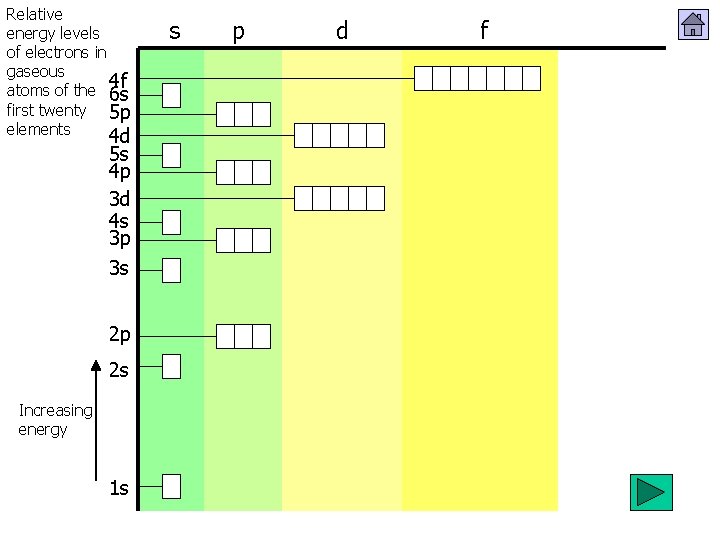
Relative energy levels of electrons in gaseous 4 f atoms of the first twenty elements 4 d 4 p 3 d 4 s 3 p 3 s 2 p 2 s Increasing energy 1 s s p d f

Relative energy levels of electrons in gaseous 4 f atoms of the 6 s first twenty 5 p elements 4 d 5 s 4 p 3 d 4 s 3 p 3 s 2 p 2 s Increasing energy 1 s s p d f

Relative energy levels of electrons in gaseous atoms of the first twenty elements s p d f Electrons fill the lowest available energy level Click to add electrons 4 p 3 d 4 s 3 p 3 s 2 p 2 s Increasing energy 1 s Cr an Cu an electron promoted from 4 s 4 s to to 4 s fills before 3 disis promoted 3 d to give a half-filled 3 d subshell full 3 d subshell Electrons remain unpaired as far as possible

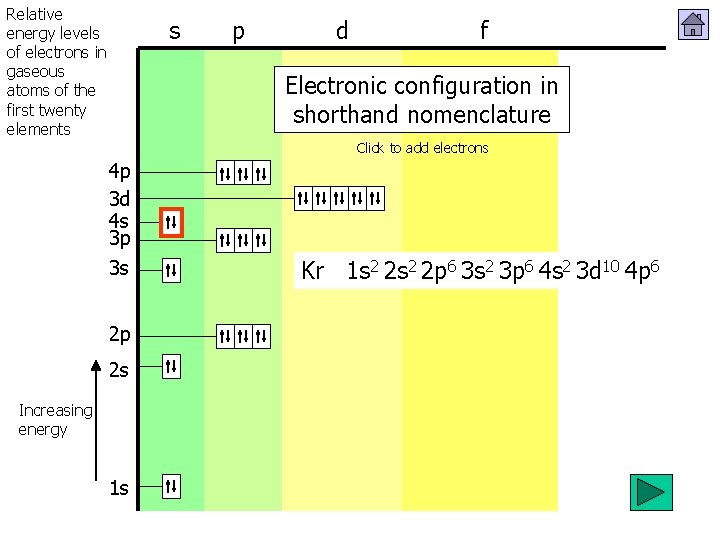
Relative energy levels of electrons in gaseous atoms of the first twenty elements s p d f Electronic configuration in shorthand nomenclature Click to add electrons 4 p 3 d 4 s 3 p 3 s 2 p 2 s Increasing energy 1 s 12222222 22 s 2 12222222 p 2 561 2346666 66 22222 12 2 3 462 6 1 56664 s 1 2221 223 d 32810 1 5 10 610 710 10 5 4 p H 1 s He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 1 s 1 s 2 s 2 s 2 s 2 p 2 p 2 p 3 s 3 s 3 s 3 p 3 p 3 p 4 s 4 s 3 d 3 d 3 d 4 p 4 p 653412

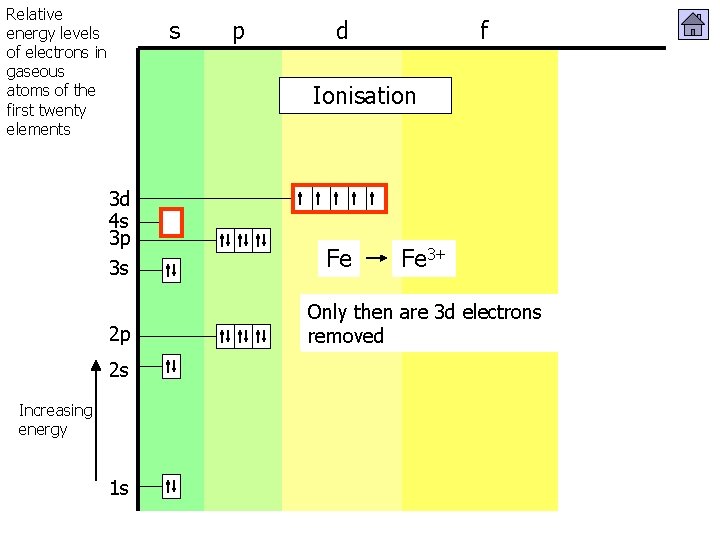
Relative energy levels of electrons in gaseous atoms of the first twenty elements s p d f Ionisation 3 d 4 s 3 p 3 s 2 p 2 s Increasing energy 1 s Zn Zn 2+ 4 s electrons (outer shell) are removed before 3 d (inner shell)

Relative energy levels of electrons in gaseous atoms of the first twenty elements s p d f Ionisation 3 d 4 s 3 p 3 s 2 p 2 s Increasing energy 1 s Fe 3+ Fe 2+ 4 s electrons shell) are Only then are(outer 3 d electrons removed before 3 d (inner shell)