Relationship Between Ka and Kb Consider the dissociation

Relationship Between Ka and Kb
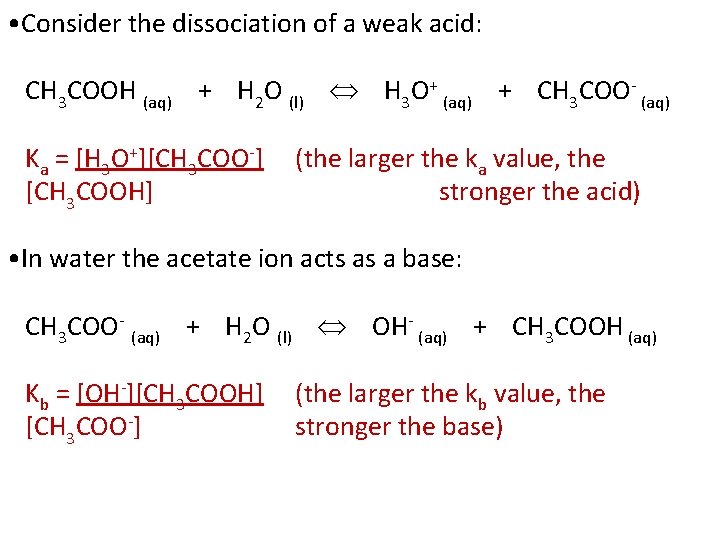
• Consider the dissociation of a weak acid: CH 3 COOH (aq) + H 2 O (l) H 3 O+ (aq) + CH 3 COO- (aq) Ka = [H 3 O+][CH 3 COO-] [CH 3 COOH] (the larger the ka value, the stronger the acid) • In water the acetate ion acts as a base: CH 3 COO- (aq) + H 2 O (l) OH- (aq) + CH 3 COOH (aq) Kb = [OH-][CH 3 COOH] [CH 3 COO-] (the larger the kb value, the stronger the base)
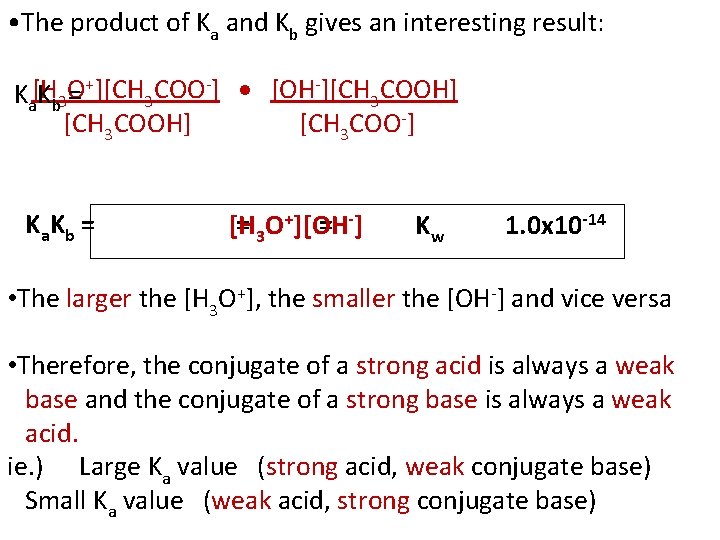
• The product of Ka and Kb gives an interesting result: Ka[H Kb 3 O = +][CH 3 COO-] [OH-][CH 3 COOH] [CH 3 COO-] K a. K b = = 3 O+][OH = -] [H Kw 1. 0 x 10 -14 • The larger the [H 3 O+], the smaller the [OH-] and vice versa • Therefore, the conjugate of a strong acid is always a weak base and the conjugate of a strong base is always a weak acid. ie. ) Large Ka value (strong acid, weak conjugate base) Small Ka value (weak acid, strong conjugate base)

Ex) Calculate the p. H of a solution that contains 12. 5 g of sodium acetate (Na. CH 3 COO) dissolved in 1. 0 L of H 2 O. Only the acetate ion affects the p. H of the solution. (Ka for acetic acid is 1. 8 x 10 -5) Na. CH 3 COO(aq) Na+(aq) + CH 3 COO-(aq) n = m/M C = n/V = (12. 5 g)/(82 g/mol) = (0. 1524 mol)/(1. 0 L) = 0. 1524 mol = 0. 1524 M CH 3 COO- i c e 0. 1524 M -x 0. 1524 - x + H 2 O CH 3 COOH 0 +x x + OH-(aq) 0 +x x
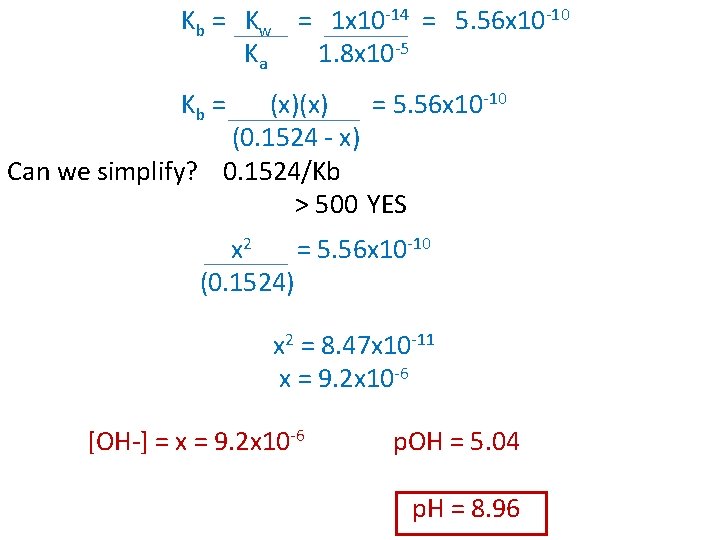
Kb = Kw = 1 x 10 -14 = 5. 56 x 10 -10 Ka 1. 8 x 10 -5 Kb = (x)(x) = 5. 56 x 10 -10 (0. 1524 - x) Can we simplify? 0. 1524/Kb > 500 YES x 2 = 5. 56 x 10 -10 (0. 1524) x 2 = 8. 47 x 10 -11 x = 9. 2 x 10 -6 [OH-] = x = 9. 2 x 10 -6 p. OH = 5. 04 p. H = 8. 96
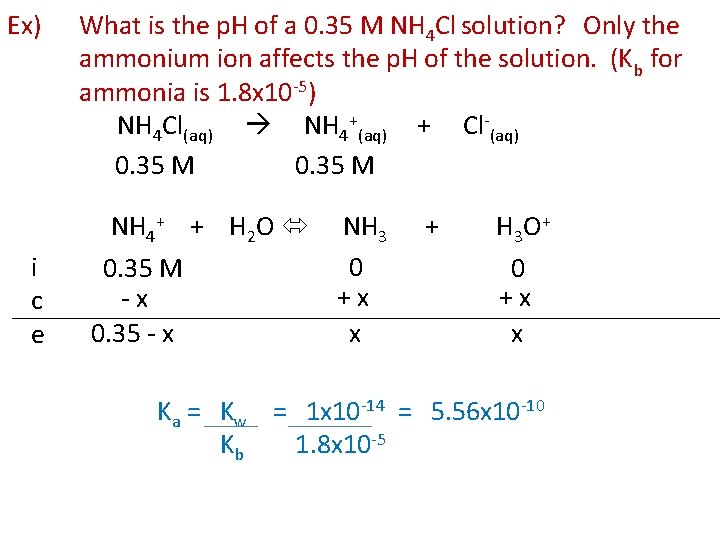
Ex) i c e What is the p. H of a 0. 35 M NH 4 Cl solution? Only the ammonium ion affects the p. H of the solution. (Kb for ammonia is 1. 8 x 10 -5) NH 4 Cl(aq) NH 4+(aq) + Cl-(aq) 0. 35 M NH 4+ + H 2 O NH 3 0 0. 35 M +x -x 0. 35 - x x + H 3 O+ 0 +x x Ka = Kw = 1 x 10 -14 = 5. 56 x 10 -10 Kb 1. 8 x 10 -5
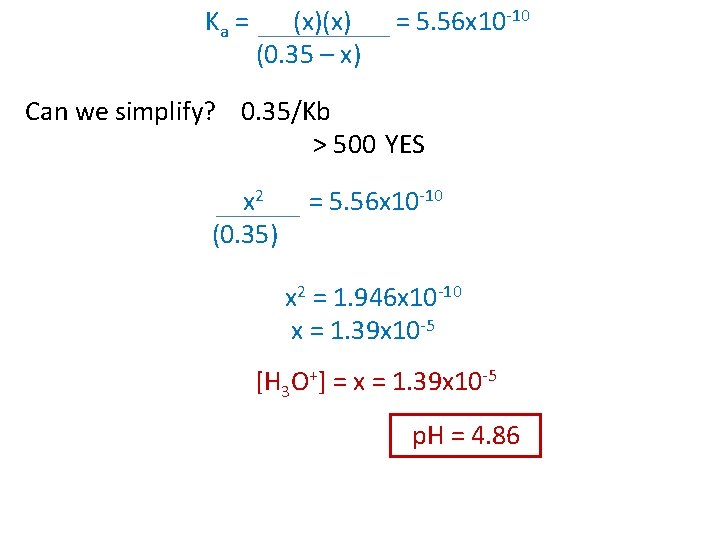
Ka = (x)(x) (0. 35 – x) = 5. 56 x 10 -10 Can we simplify? 0. 35/Kb > 500 YES x 2 (0. 35) = 5. 56 x 10 -10 x 2 = 1. 946 x 10 -10 x = 1. 39 x 10 -5 [H 3 O+] = x = 1. 39 x 10 -5 p. H = 4. 86

HOMEWORK p 409 #35 -38
- Slides: 8