Regulation of the Immune Response The immune response



- Slides: 25

Regulation of the Immune Response • The immune response is subject to a variety of control mechanisms which serve to restore the immune system to a resting state when the response to a given antigen is no longer required. • The nature of the immune response is determined by many factors, including the form and route of administration of the antigen, APC, the genetic background of the individual, and any history of previous exposure to the antigen in question or to a cross-reacting antigen. Specific antibodies may also modulate the immune response to an antigen.

A- Regulation by Antigen: 1. The nature of the antigen Different antigens elicit different kinds of immune response. Polysaccharide capsule antigens of bacteria generally induce Ig. M responses, whereas proteins can induce both cell-mediated and humoral immune responses. Intracellular organisms induce a cell-mediated immune response, whereas soluble proteins induce a humoral response. A cell-mediated immune response is also induced by agents such as silica.

2. Antigen Dose Very large doses of antigen often result in specific T and sometimes B-Cell tolerance. • Administration of antigen to neonatal mice often results in tolerance to this antigen, it may be the result of the immaturity of the immune system. Recent studies have shown that neonatal mice can develop efficient immune responses. • TI polysaccharide antigens generate tolerance in B cells after administration in high doses.

3. The route of administration of an antigen • The route of administration of an antigen influences the immune response. • Subcutaneous or intradermal administration evoke an immune response, whereas intravenous, oral or an aerosol may cause tolerance. • Mice primed subcutaneously with peptide in incomplete Freund's adjuvant develop immunity to lymphocytic choriomeningitis virus "LCMV", however, if the same peptide is repeatedly injected intraperitoneally, the animal becomes tolerized and cannot clear the virus.

B- The antigen Presenting Cell "APC" • Presentation by dendritic cells or activated macrophages, which express high levels of MHC class II in addition to costimulatory molecules, results in highly effective T-cell activation. • The interaction of CD 40 L on activated T cells with CD 40 on dendritic cells is important for production of IL-12 which is necessary for the generation of an effective TH-1 response. • If antigen is presented to T cells by a non-professional APC that is unable to provide co-stimulation, then unresponsiveness or immune deviation results. • Adjuvants may facilitate immune response by inducing expression of high levels of MHC and co-stimulatory molecules on APCs.

C- Regulation by Antibody • Antibody has been shown to exert feedback control on the immune response. Passive administration of Ig. M antibody together with an antigen specifically enhances the immune response to that antigen, whereas Ig. G antibody suppresses the response. • Mumps and measles vaccines are given to infants by the end of the first year, because levels of maternally derived Ig. G remain high for at least 6 months after birth. These Ig. G antibodies would cause an inadequate immune response. • In Rh incompatibility, anti Rh. D antibody is given to neutralize the foreign antigen "fetal RBCs". • Antibody present blocks the interaction between an antigenic determinant "epitope" and membrane Ig on B cells. The B cell is then unable to recognize the antigen.

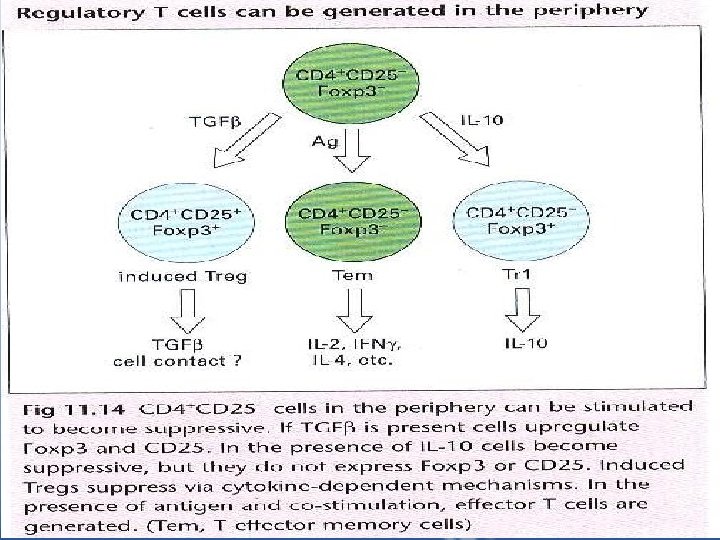
D- Regulation by Lymphocytes • T cells modulate the immune response in a positive sense by providing T-cell help. The kind of help "TH 1 or TH 2" affects the nature of the immune response, favoring either humoral or cell-mediated immunity. • T cells are capable of downregulating immune responses. • There is a good evidence of the presence of other CD 4+ T cell subsets; Terg(CD 4+ CD 25+), Tr 1, TH 3 and Tem(Fig. 11. 14) which are able to regulate immune responses. These differ in cytokine production; Treg secrets TGFβ, Tr 1 secrets IL-10, while Tem secrets IL-2, IFNγ, IL-4


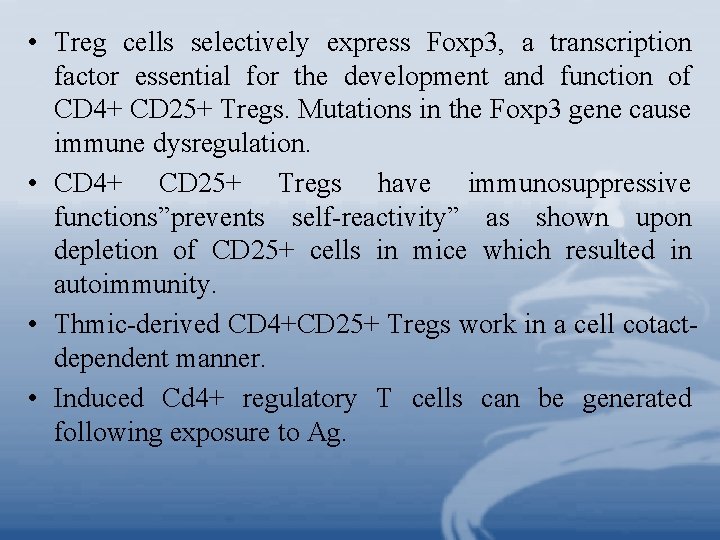
• Treg cells selectively express Foxp 3, a transcription factor essential for the development and function of CD 4+ CD 25+ Tregs. Mutations in the Foxp 3 gene cause immune dysregulation. • CD 4+ CD 25+ Tregs have immunosuppressive functions”prevents self-reactivity” as shown upon depletion of CD 25+ cells in mice which resulted in autoimmunity. • Thmic-derived CD 4+CD 25+ Tregs work in a cell cotactdependent manner. • Induced Cd 4+ regulatory T cells can be generated following exposure to Ag.

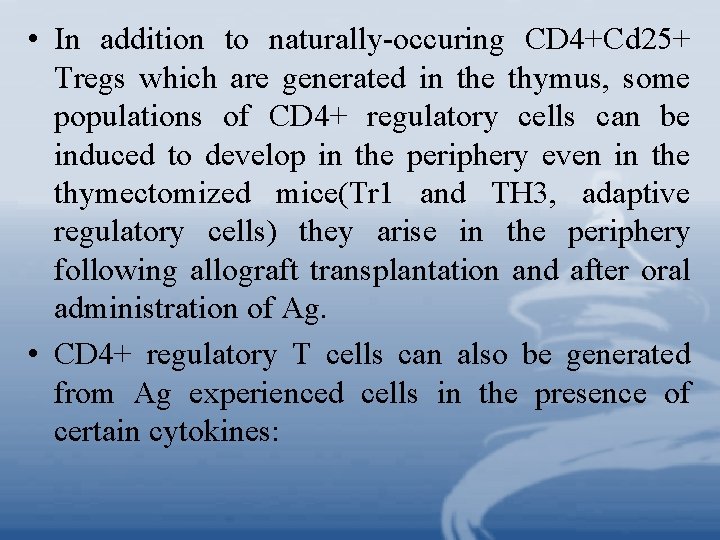
• In addition to naturally-occuring CD 4+Cd 25+ Tregs which are generated in the thymus, some populations of CD 4+ regulatory cells can be induced to develop in the periphery even in the thymectomized mice(Tr 1 and TH 3, adaptive regulatory cells) they arise in the periphery following allograft transplantation and after oral administration of Ag. • CD 4+ regulatory T cells can also be generated from Ag experienced cells in the presence of certain cytokines:

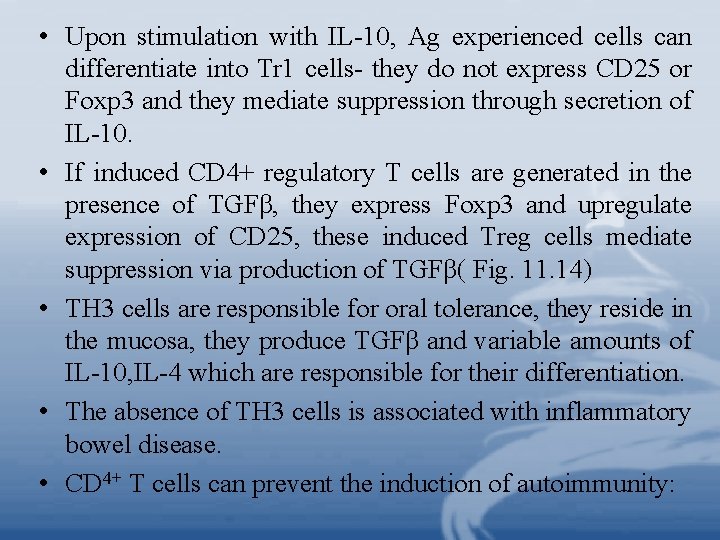
• Upon stimulation with IL-10, Ag experienced cells can differentiate into Tr 1 cells- they do not express CD 25 or Foxp 3 and they mediate suppression through secretion of IL-10. • If induced CD 4+ regulatory T cells are generated in the presence of TGFβ, they express Foxp 3 and upregulate expression of CD 25, these induced Treg cells mediate suppression via production of TGFβ( Fig. 11. 14) • TH 3 cells are responsible for oral tolerance, they reside in the mucosa, they produce TGFβ and variable amounts of IL-10, IL-4 which are responsible for their differentiation. • The absence of TH 3 cells is associated with inflammatory bowel disease. • CD 4+ T cells can prevent the induction of autoimmunity:

1. The administration of high doses of autoantigen prevents induction of autoimmunity. 2. This inhibition is due to CD 4+T cells which inhibit the development of autoantibodies as well as the autoimmune disease. 3. It is suggested that the suppression may be due to the involvement of TGF and IL-10. Ø TH subsets are involved in the regulation of Ig production: § IFN secreted by TH 1 cells can inhibit the responsiveness of TH 2 cells. § IL-10 produced by TH 2 cells downregulates B 7 and IL-12 expression by APC, which in turn inhibits TH 1 activation.

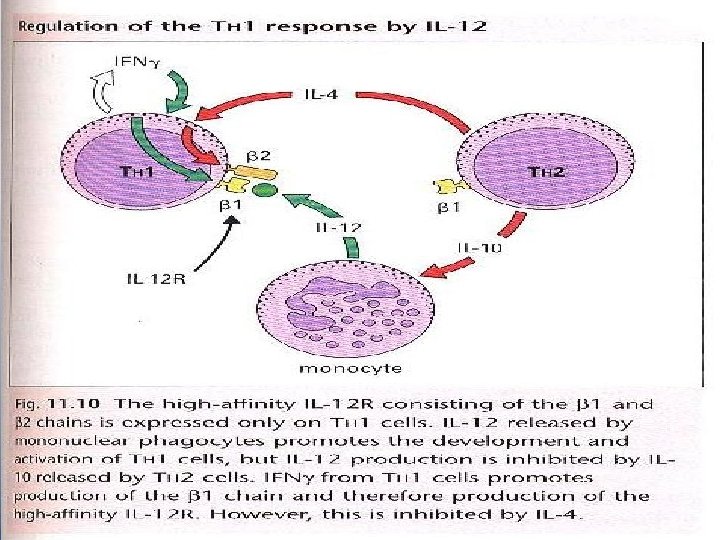
§ IL-12 is important in the development of TH 1 responses and the TH 1/TH 2 balance is modulated by IL-12 and the expression of IL 12 receptor. § T cell subset development is influenced by IFN , which favors TH 1 development, even in the presence of IL-4 and neutralization of IL-12. thus the preferential activation of TH 1 or TH 2 cells may result in an immune deviation. "Fig 11. 10”CD 8+ T cells can transfer resistance and tolerance, this effect is thought to be mediated by TGF.


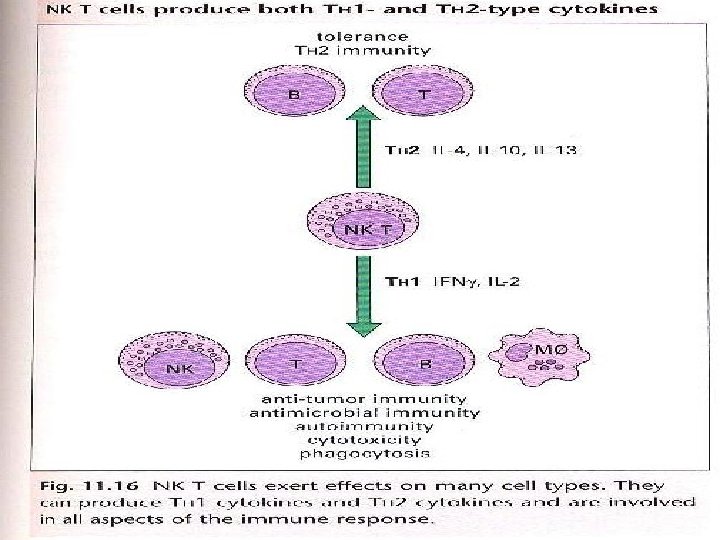
E- Regulation by NK and NK T cells • NK cells play a key role in the early immune response to intracellular pathogens, largely through their production of IFN which activates macrophages and facilitate differentiation of TH 1 cells. • NK cell activity itself is induced by a variety of cytokines including IFN , IL-15, IL-18, IL-12. • NK cells are negatively regulated by cytokines such as IL-10 and TGF. • NK T cells secreting IFN are able to induce NK cell activation, increasing both NK proliferation and cytotoxicity. • The ability of NK T cells to secrete IL-4 particularly in the thymus has been associated with the prevention of autoimmunity. • Defeciencies in NK T cells have also been reported in humans autoimmune diseases including rheumatoid arthritis, psoriasis, ulcerative colitis and multiple


F- Regulation by Localization of cells • The production of chemokines by different cell types is an important mechanism of immune regulation. • The expression of different chemokine receptors on TH 1 cells "CXCR 3 and CCR 5" and TH 2 cells "CCR 3, CCR 4, CCR 8" allow chemotactic signals to produce the differential localization of T-cell subsets to sites of inflammation. • The production of MCP-1, MIP-1 , IP-10 and RANTES serves to focus mononuclear phagocytes to the area of inflammation. • The ability of cytokines such as TGF , IL-12 and IL-4 to influence chemokine or chemokine receptor expression provides a further level of control on cell migration and recruitment. • Several viruses evade the host immune response by making chemokine receptor antagonists.

Idiotype Modulation of Responses • Tolerance to self antigens in established during ontogeny. • Tolerance may develop to FC portions of antibody molecules but not to Fab region. • Antibodies formed against Fab are called anti-idiotypic antibodies and are capable of influencing the outcome of an immune response. • Immunogenic epitopes in or around the binding site are termed idiotopes. • Jerne proposed that an immune network existed within the body which interacted by means of idiotype recognition. • When antibody response is induced by antigen, this antibody inturn, will evoke an anti-idiotypic response to itself.

Neuroendocrine Modulation of Immune Responses • There is evidence that the nervous, endocrine and immune systems are interconnected. A. Most lymphoid tissues receive direct sympathetic innervations. B. The nervous system controls the output of various hormones e. g corticosteriods, GH, prolactin, thyroxine and epinephrine. C. Lymphocytes express receptors for many hormones. D. Corticosteroids act as a major feedback control on immune responses. They inhibit TH 1 cytokine production they induce the production of TGF which in turn may inhibit the immune response.

Genetic Control of Immune Responses There are several ways in which genes influence the immune response: § MHC haplotypes influence the ability to respond to an antigen • Strains of mice with different MHC haplotypes vary in their ability to mount an antibody response to specific antigens. this function depends on MHC class II molecules, and is specific for each antigen. Genes within MHC play a fundamental role against infectious agents. • The extensive sequence polymorphism of MHC molecules has a deep impact on peptide binding and, as a consequence, on T cell activation.

• MHC genes have a major influence on susceptibility to autoimmune diseases § Insulin-dependant diabetes mellitus "IDDM", an autoimmune disease in which the beta cells of the pancreas are destroyed by cells of the immune system, is associated with HLA-DR 3 and HLA-DR 4. the high risk is seen in HLADR 3/4 heterozygotes. § Other MHC-linked genes are involved in proteolysis and transport of antigen peptides to MHC molecules for presentation to cells of the immune system.

• Many non-MHC genes also modulate immune responses § These genes are less polymorphic than MHC genes. Their effects have been clearly shown in autoimmune diseases, allergy and infection. Examples: • Individuals with defects in the complement components clq, clr, cls are predisposed to develop SLE. Deficiency in C 3 leads to an increased susceptibility to bacterial infection and predisposition to immune-complex disease. • High Ig. E production in some allergy-prone families is due to "atopy gene" or human chromosome llq.

• Non-MHC linked genes affect susceptibility to infection § Macrophage activities are regulated by specific genes. § Lsh/Ity/Beg gene governs the early response to infection with Mycobacterium and Salmonella. The response includes: • Upregulation of the oxidative burst. • Enhanced tumoricidal activity. • Enhanced antimicrobial activity. • Upregulation of MHC class II expression.

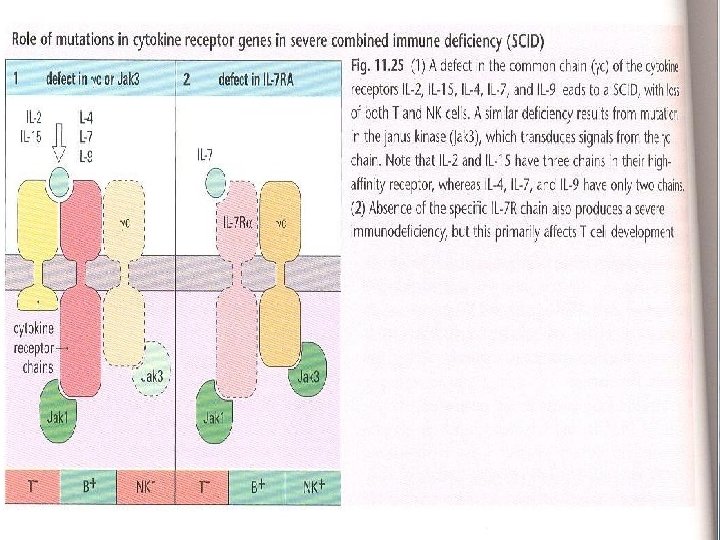
• Polymorphism in the genes encoding cytokine receptors have been shown to correlate with an increased susceptibility to infection, Severe Combined Immunodeficiency "SCID" or inflammatory conditions. • The outcome of the mutation is dependent on the cytokine gene which is affected. For example, humans with mutations in the IL-7 R develop a selective deficit in T cells and those with deficiency in the common cytokine receptor chain " C", which is a component of the functional receptor for IL-2, IL 4, IL-7, IL-9 and IL-15 have reduced numbers of T cells and NK cells and have impaired B cell function, in part due to the lack of T cell help. Fig 11 -25
