Region II Infertility Prevention Project New York City











- Slides: 12

Region II Infertility Prevention Project New York City, New York May 31, 2006 Richard Steece, Ph. D. , D(ABMM) National Infertility Prevention Project Dr. RSteece@aol. com

Region II Infertility Prevention Project New York City, New York May 31, 2006 Supplemental Testing (Repeat Testing) Alternate Specimens (vaginal, rectal and pharyngeal) CDC/APHL 2004 National STD Laboratory Survey

Supplemental Testing • Zanto, et. al. ; Region VIII – Gen-Probe ACT and AGC reagents can be used effectively as a supplemental test – Supplemental testing (repeat Gen-Probe AC 2) is equally as effective as an alternate target (ACT) – Reporting the initial positive AC 2 screening result in this low prevalence (7. 5%) population had a greater than 95% positive predictive value (PPV) for CT – Reporting the initial positive AC 2 screening result in this low prevalence (1. 6%) population had a 91. 3% PPV for GC – Based on these PPVs, supplemental or repeat testing need not be routinely performed with NAAT screening, resulting in cost savings.

Alternate Specimens (vaginal, rectal and pharyngeal) • • References California Action Coalition (CAA) CDC/FDA – Dr. Papp Workshop NIAID (June 28 -29, 2006)

Sexually Transmitted Diseases Laboratory Survey, 2004

Methods: Laboratory Survey • Surveyed 162 public, private and university laboratories in February 2005 – All members of the Association of Public Health Laboratories (APHL) – All laboratories that participate in CDC’s Regional Infertility Prevention Project • An on-line survey was developed that requested the following information for calendar year 2004: – Volume and type of testing for chlamydia and gonorrhea – Volume and type of testing for HSV, Trichomonas, Syphilis, Bacterial Vaginosis, HPV, and Pap Smears

Methods: Laboratory Survey • An e-mail was sent to each laboratory director with link to survey – 34/162 e-mails not successfully transmitted; corrected some addresses and resent. – 25/34 e-mails failed to transmit again; FAXes sent to those lab directors with the link to the survey. • Asked for responses in two weeks • Non-responder follow-up – 2 follow-up e-mails were sent at the end of weeks 1 & 2 – Additional e-mails and phone calls were made after 3 weeks

Results: Laboratory Survey Response • Survey was completed by 119 laboratories (73% response rate) • Responding laboratories were from 49 states and two U. S. territories: – 51% state laboratories (n=61) – 34% county laboratories (n=41) – 10% city laboratories (n=12) – 2% U. S. territory laboratories (n=2) – 2% university laboratories (n=2) – 1% private laboratories (n=1) • Focused on public health laboratories (95% of responding laboratories) • Survey was completed by 114 of 144 public health laboratories (79% response rate)

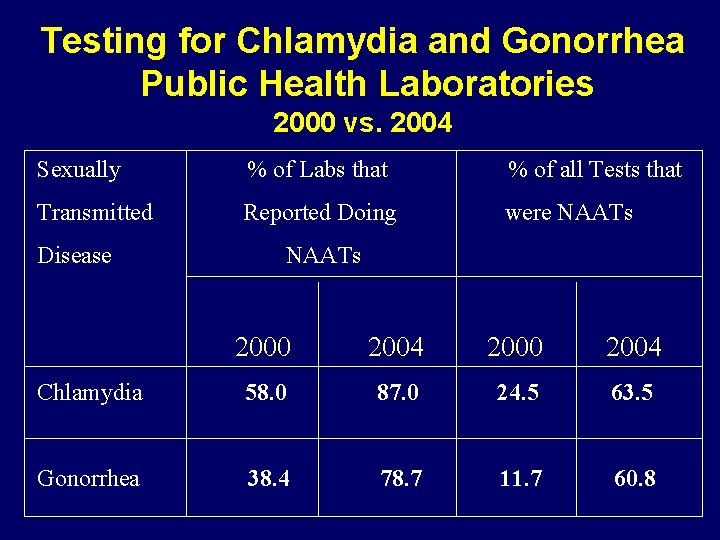
Testing for Chlamydia and Gonorrhea Public Health Laboratories 2000 vs. 2004 Sexually % of Labs that % of all Tests that Transmitted Reported Doing were NAATs Disease NAATs 2000 2004 Chlamydia 58. 0 87. 0 24. 5 63. 5 Gonorrhea 38. 4 78. 7 11. 7 60. 8

Status of Gonorrhea Culture Public Health Laboratories 2000 vs. 2004 Sexually % of Labs that % of all Tests done Transmitted Reported Doing that were Culture Disease Gonorrhea GC Culture 2000 2004 79. 8 77. 8 18. 0 8. 5

Laboratory Survey: Next Steps • Compiled and distributed survey results – Available via the APHL website – Distributed to IPP Coordinators (Word) • Chlamydia and Gonorrhea data was presented in a poster at ISSTDR, July 2005 • Collected information from test kit manufacturers – Number of CT & GC test kits sold in 2004 – % or # sold to public health laboratories • Prepared and submitted manuscript on survey results to JSTD (July)

Male Chlamydia Screening Consultation March 28 -29, 2006 • Urine is the specimen of choice when screening males (NAATS) • Leukocyte Esterase Test (LET) is not recommended in any venue. • Pooling should strongly be considered in low prevalence populations (<10%).
 Primary prevention secondary prevention tertiary prevention
Primary prevention secondary prevention tertiary prevention Hunger in new york city poem
Hunger in new york city poem Why is nyc called the big apple
Why is nyc called the big apple New york city technology forum
New york city technology forum New york city department of youth and community development
New york city department of youth and community development Sappi fine paper
Sappi fine paper Siting
Siting Event new york city
Event new york city Research alliance for new york city schools
Research alliance for new york city schools Pmi new york city
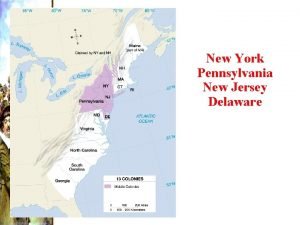
Pmi new york city New york pennsylvania new jersey delaware
New york pennsylvania new jersey delaware Marquee cinemas - orchard 10
Marquee cinemas - orchard 10 Articles of confederation characteristics
Articles of confederation characteristics