Regents Physics Unit VI Work Power Energy PART


















































- Slides: 77

Regents Physics Unit VI: Work, Power & Energy

PART I: WORK

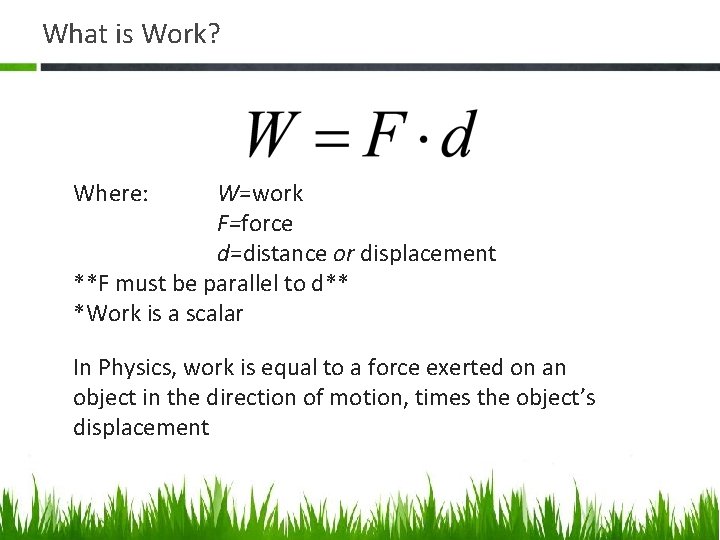
What is Work? Where: W=work F=force d=distance or displacement **F must be parallel to d** *Work is a scalar In Physics, work is equal to a force exerted on an object in the direction of motion, times the object’s displacement

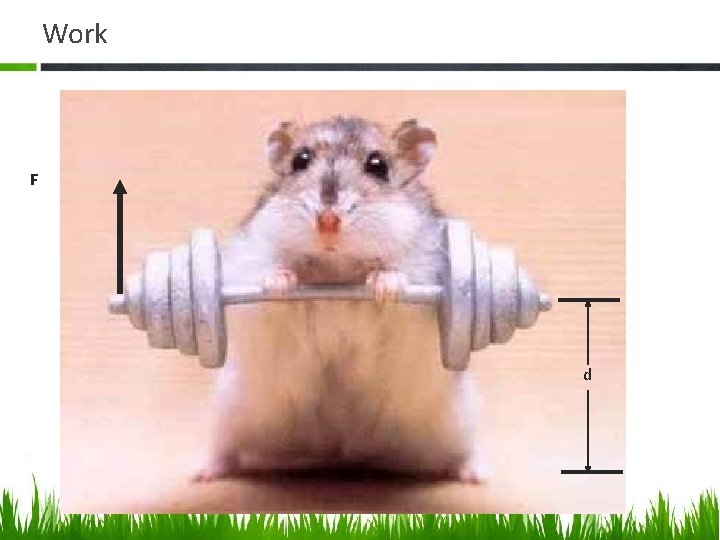
Work F d

“Spinning Your Wheels…” • In other words, if the object doesn’t move, you do no work.


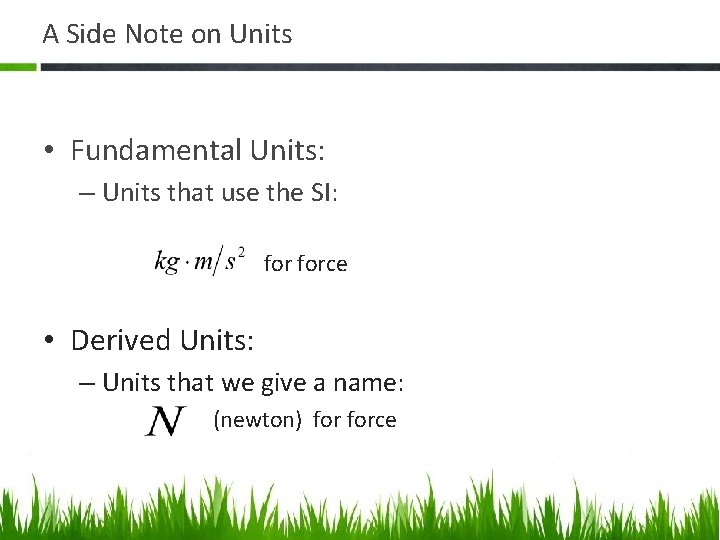
A Side Note on Units • Fundamental Units: – Units that use the SI: • F force • Derived Units: – Units that we give a name: (newton) force

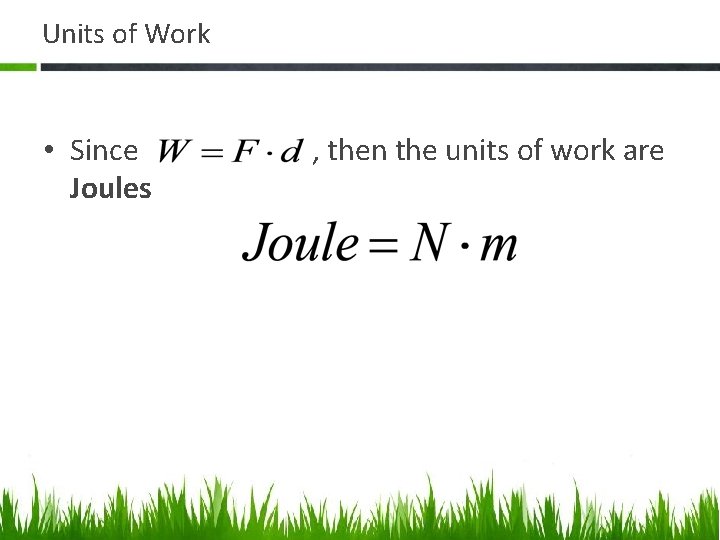
Units of Work • Since Joules , then the units of work are

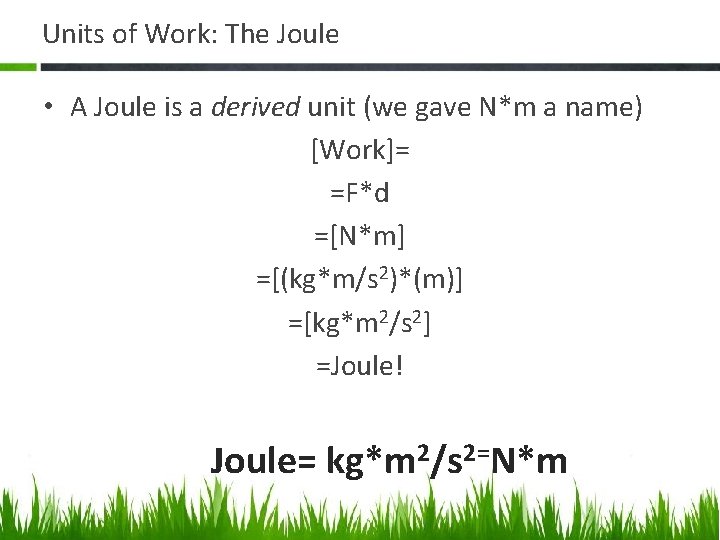
Units of Work: The Joule • A Joule is a derived unit (we gave N*m a name) [Work]= =F*d =[N*m] =[(kg*m/s 2)*(m)] =[kg*m 2/s 2] =Joule! Joule= kg*m 2/s 2=N*m

What is a Joule? • The Joule (SI units) is equivalent to the Calorie (pre-SI units). – *There are kilocalories (capital C) and calories (lower case c). • You intake “Calories” every day. Without eating you would be weak and you wouldn’t have much energy… • It turns out that the Joule(Calorie/calorie) is a unit of Energy.

What is a Joule? (Cont’d) • One “food” Calorie = 4, 180 Joules - A Snickers bar has about 280 Calories, or 1, 170, 400 Joules. - So…Doing “Work” on something changes its total energy. - We’ll come back to this later.

Work: An example problem A 105 kg hockey puck is sliding across the ice. A player exerts a constant 4. 5 N force over a distance of 0. 150 m. How much work does the player do on the puck?

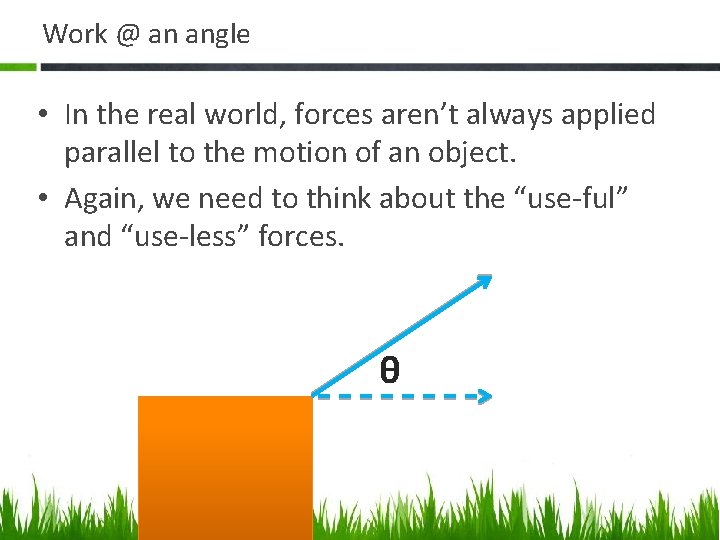
Work @ an angle • In the real world, forces aren’t always applied parallel to the motion of an object. • Again, we need to think about the “use-ful” and “use-less” forces. θ

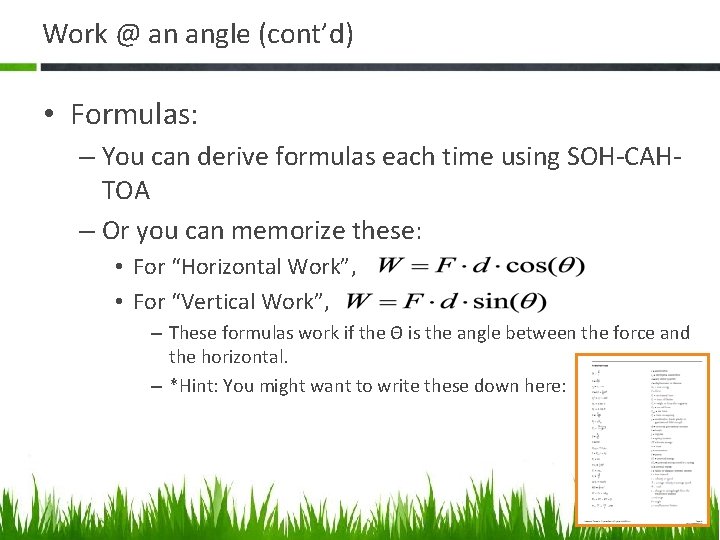
Work @ an angle (cont’d) • Formulas: – You can derive formulas each time using SOH-CAHTOA – Or you can memorize these: • For “Horizontal Work”, • For “Vertical Work”, – These formulas work if the Θ is the angle between the force and the horizontal. – *Hint: You might want to write these down here:

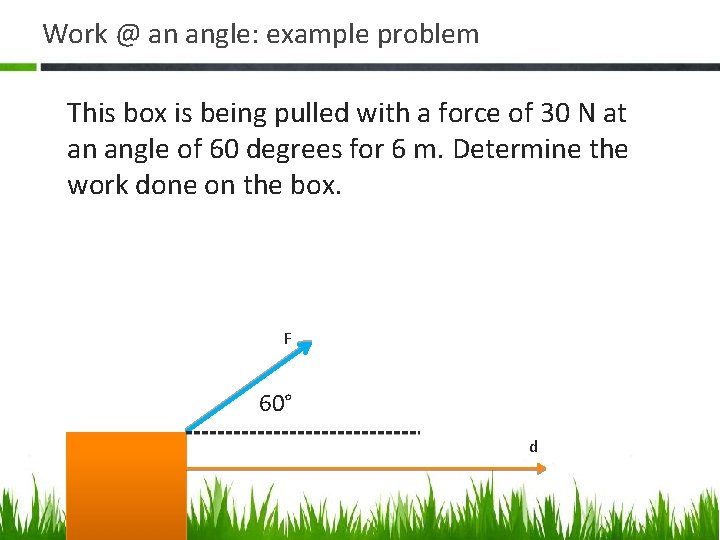
Work @ an angle: example problem This box is being pulled with a force of 30 N at an angle of 60 degrees for 6 m. Determine the work done on the box. F 60° d

PART II: POWER

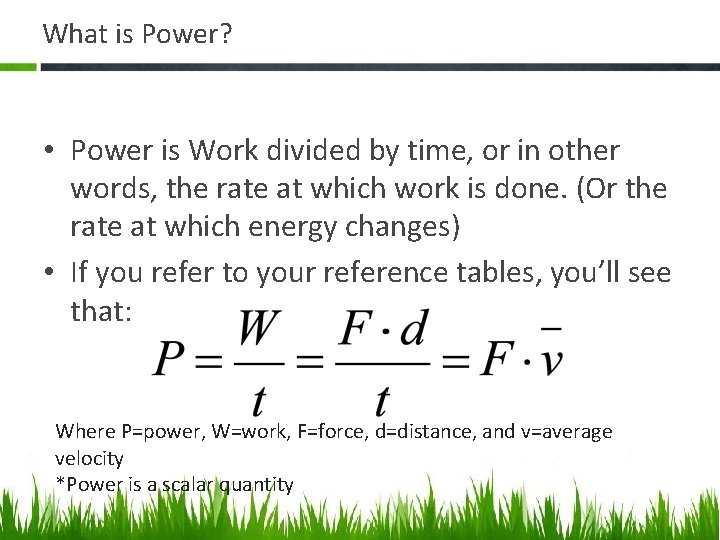
What is Power? • Power is Work divided by time, or in other words, the rate at which work is done. (Or the rate at which energy changes) • If you refer to your reference tables, you’ll see that: Where P=power, W=work, F=force, d=distance, and v=average velocity *Power is a scalar quantity


Units of Power • The unit of Power is the Watt. – We use the letter W to represent the unit Watt (don’t confuse it with W for work on the reference tables) Watt=Joules/seconds Watt? Don’t worry, Joule get it in a second!

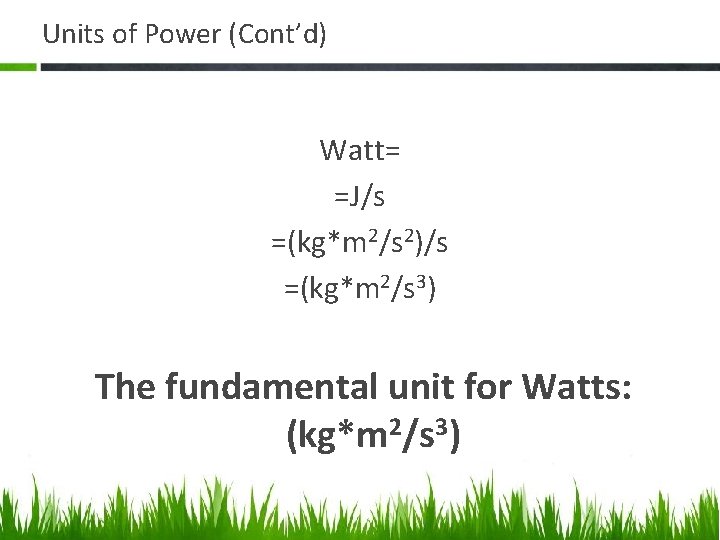
Units of Power (Cont’d) Watt= =J/s =(kg*m 2/s 2)/s =(kg*m 2/s 3) The fundamental unit for Watts: (kg*m 2/s 3)

Power Example Problem: • When Justin Beiber lifts his love child, 1400 J of work is done in 2. 5 s. Calculate how much power Justin develops.

Another Power Example Problem… • Ndamukong Suh stomps on a player with a force of 750 N at an angle of 45 degrees. He pushes the player a distance of 1. 5 m in. 5 seconds. How much power did he deliver to the player?

Energy: Forms and Changes

The Nature of Energy is all around you! n n n You can hear energy as sound. You can see energy as light. And you can feel it as wind.

Nature of Energy You use energy when you: n n n hit a softball. lift your book bag. compress a spring.

Nature of Energy What is energy? How can it be involved in so many different activities? n n Energy can be defined as the ability to do work. If an object or organism does work (exerts a force over a distance to move an object) the object or organism uses energy.

Nature of Energy Because of the direct connection between energy and work, energy is measured in the same unit as work: joules (J). In addition to using energy to do work, objects gain energy because work is being done on them.

Forms of Energy The five main forms of energy are: n n n Heat Chemical Electromagnetic Nuclear Mechanical

Heat Energy The internal motion of the atoms is called heat energy, because moving particles produce heat. Heat energy can be produced by friction. Heat energy causes changes in temperature and phase of any form of matter. Heat energy is symbolized by the letter Q (or for the chemists-q. )

Chemical Energy is required to bond atoms together. When bonds are broken, energy is released. Fuel and food are forms of stored chemical energy.

Electromagnetic Energy Light is a form of electromagnetic energy. Each color of light (Roy G Bv) represents a different amount of electromagnetic energy. Electromagnetic Energy is also carried by X-rays, radio waves, and laser light.

Nuclear Energy The nucleus of an atom is the source of nuclear energy.

Nuclear Energy The sun’s energy is produced from a nuclear fusion reaction in which hydrogen nuclei fuse to form helium nuclei.

Nuclear Energy When the nucleus splits (fission), nuclear energy is released in the form of heat energy and light energy. Nuclear energy is also released when nuclei collide at high speeds and join (fuse). Nuclear energy is the most concentrated form of energy.

Mechanical Energy When work is done to an object, it acquires energy. The energy it acquires is known as mechanical energy.

Mechanical Energy When you kick a football, you give mechancal energy to the football to make it move.

Mechanical Energy When you throw a bowling ball, you give it energy. When that bowling ball hits the pins, some of the energy is transferred to the pins.

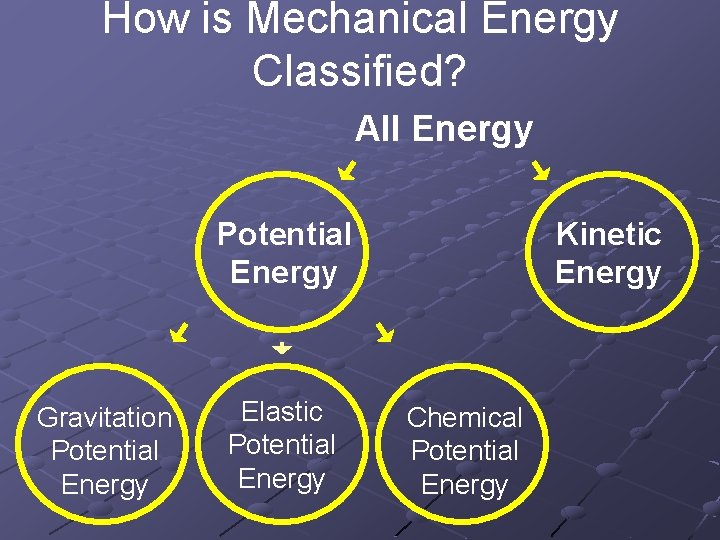
How is Mechanical Energy Classified? All Energy Potential Energy Gravitation Potential Energy Elastic Potential Energy Kinetic Energy Chemical Potential Energy

What is Potential Energy? o Energy that is stored and waiting to be used later

What is Gravitational Potential Energy? o Potential energy due to an object’s position Don’t look down, Rover! Good boy!

What is Elastic Potential Energy? o Potential energy due to the compression or expansion of an elastic object. Notice the ball compressing and expanding

What is Chemical Potential Energy? o Potential energy stored within the chemical bonds of an object

What is Kinetic Energy? o Energy an object has due to its motion.

More on Potential Energy is stored energy. n n Stored chemically in fuel, the nucleus of atom, and in foods. Or stored because of the work done on it: Stretching a rubber band. Winding a watch. Pulling back on a bow’s arrow. Lifting a brick high in the air.

Gravitational Potential Energy Potential energy that is dependent on height is called gravitational potential energy. A waterfall, a suspension bridge, and a snowflake about to fall have gravitational potential energy.

Gravitational Potential Energy If you stand on a 3 meter diving board, you have 3 times the G. P. E, than you had on a 1 -meter diving board.

Gravitational Potential Energy – An object gains potential energy when you lift it off the ground – An increase in potential energy is equal to the work done on the system – It also depends on the mass of the object and the height it reaches

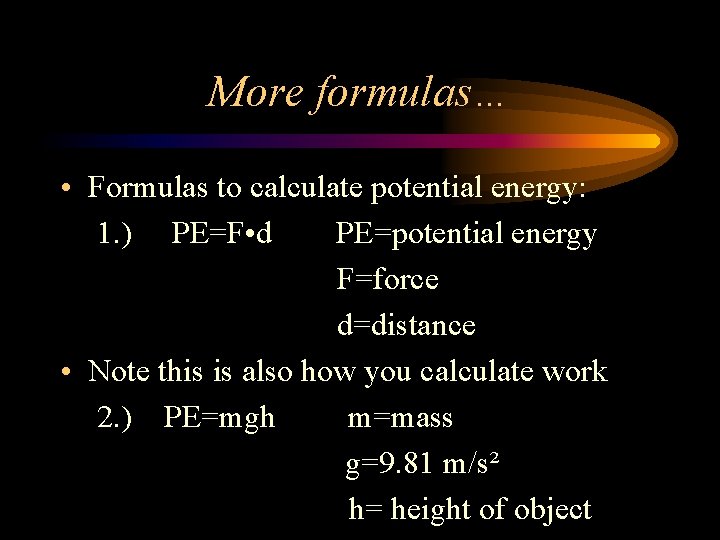
More formulas… • Formulas to calculate potential energy: 1. ) PE=F • d PE=potential energy F=force d=distance • Note this is also how you calculate work 2. ) PE=mgh m=mass g=9. 81 m/s² h= height of object

Sample Problem • A plane flies at an altitude of 12, 500 m, then descends to 6000 m. If the plane weighs 42, 000 N, how much potential energy did the plane lose on the dive?

Another sample! • A rocket is launched with a force of 4 6 x 10 N as it covers a distance of 1 x 103 m. • How much potential energy does the rocket have?

Stretch & get energy!! Elastic Potential Energy – Defined as a special type of potential energy generated when springs are compressed or stretched out. – In order to calculate the potential energy, we need to know a spring’s “Spring Constant” (k)

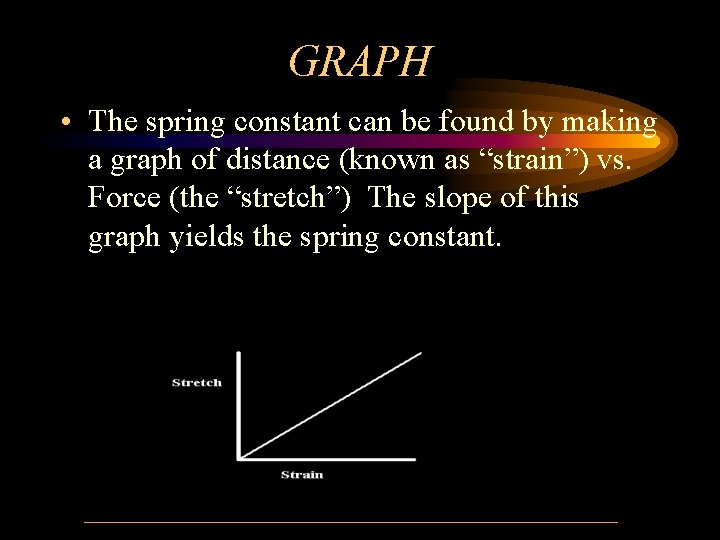
GRAPH • The spring constant can be found by making a graph of distance (known as “strain”) vs. Force (the “stretch”) The slope of this graph yields the spring constant.

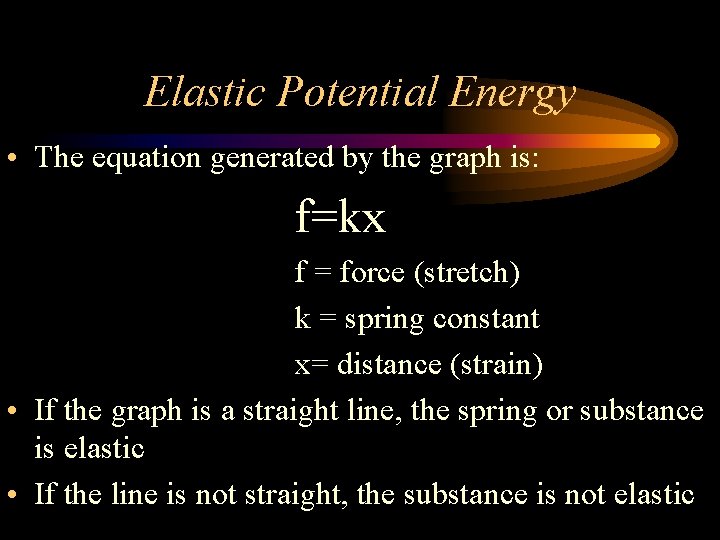
Elastic Potential Energy • The equation generated by the graph is: f=kx f = force (stretch) k = spring constant x= distance (strain) • If the graph is a straight line, the spring or substance is elastic • If the line is not straight, the substance is not elastic

Sample Problem • Sample Problem: A spring is stretched with a force of 10 N over a distance of. 5 m. What is the spring constant?

PE for a Spring • Springs may also store potential energy. This may be calculated using the formula: PE=1/2 kx 2

Sample Problem • A spring is compressed with a force of 6 N a distance of. 2 m. How much potential energy does the spring have? • Springs may be “ruined” by overstretching them. If this happens to a spring, we say it has been stretched past its elastic limit

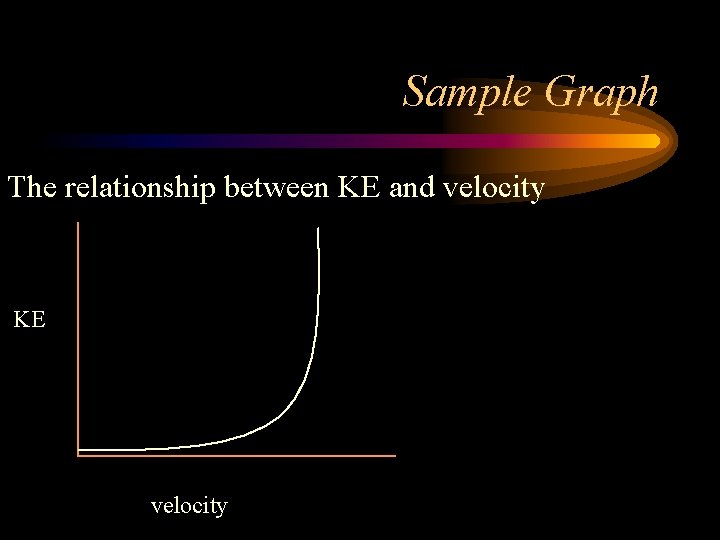
Kinetic Energy The energy of motion is called kinetic energy. The faster an object moves, the more kinetic energy it has. The greater the mass of a moving object, the more kinetic energy it has. Kinetic energy depends on both mass and velocity.

More on Kinetic Energy – The opposite of potential energy, it is the energy an object has because of its motion – When you have done work, you have lost kinetic energy – The kinetic energy depends on both the mass and the velocity of the object: – KE=1/2 mv 2 –KE = kinetic energy –m = mass –v = velocity

Sample Graph The relationship between KE and velocity KE velocity

Energy Conversion Energy can be changed from one form to another. Changes in the form of energy are called energy conversions. All forms of energy can be converted into other forms. n n The sun’s energy through solar cells can be converted directly into electricity. Green plants convert the sun’s energy (electromagnetic) into starches and sugars (chemical energy).

Other energy conversions In an electric motor, electromagnetic energy is converted to mechanical energy. n In a battery, chemical energy is converted into electromagnetic energy. n The mechanical energy of a waterfall is converted to electrical energy in a generator. n

Energy Conversions In an automobile engine, fuel is burned to convert chemical energy into heat energy. The heat energy is then changed into mechanical energy.

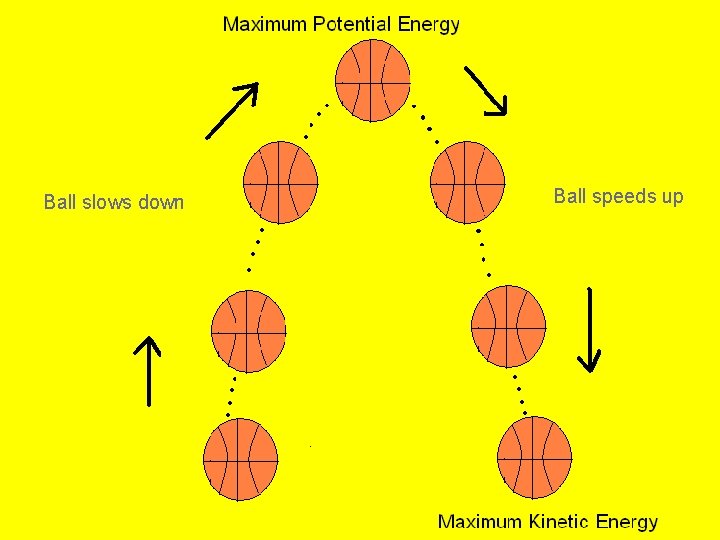
Kinetic-Potential Energy Conversion Roller coasters work because of the energy that is built into the system. Initially, the cars are pulled mechanically up the tallest hill, giving them a great deal of potential energy. From that point, the conversion between potential and kinetic energy powers the cars throughout the entire ride.

Kinetic-Potential Energy Conversions As a basketball player throws the ball into the air, various energy conversions take place.

Ball slows down Ball speeds up

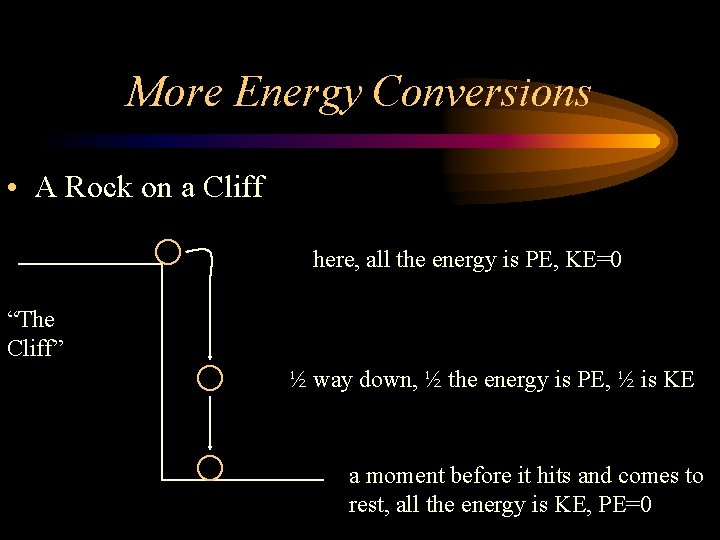
More Energy Conversions • A Rock on a Cliff here, all the energy is PE, KE=0 “The Cliff” ½ way down, ½ the energy is PE, ½ is KE a moment before it hits and comes to rest, all the energy is KE, PE=0

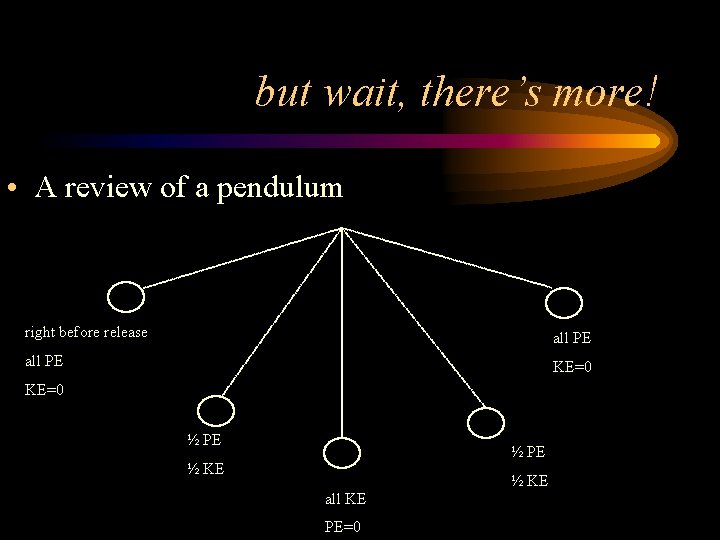
but wait, there’s more! • A review of a pendulum right before release all PE KE=0 ½ PE ½ KE all KE PE=0

The Law of Conservation of Energy can be neither created nor destroyed by ordinary means. n n It can only be converted from one form to another. If energy seems to disappear, then scientists look for it – leading to many important discoveries.

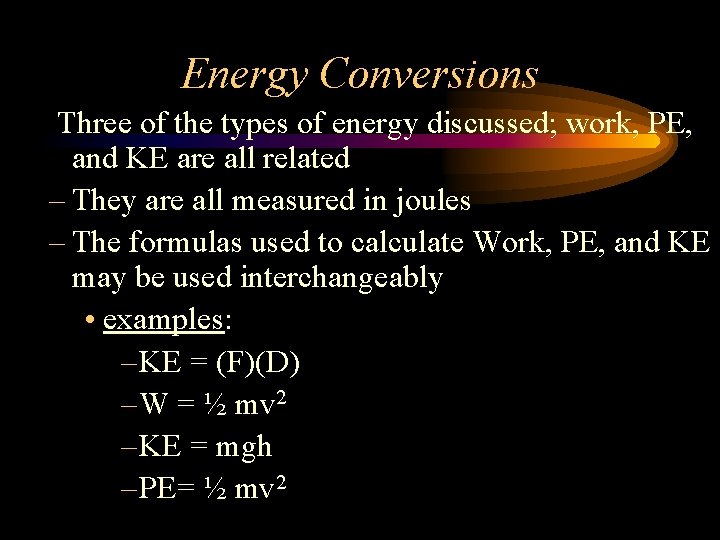
Energy Conversions Three of the types of energy discussed; work, PE, and KE are all related – They are all measured in joules – The formulas used to calculate Work, PE, and KE may be used interchangeably • examples: – KE = (F)(D) – W = ½ mv 2 – KE = mgh – PE= ½ mv 2

Sample problems to test your learning An object of mass 26 kg moves 26 m in 2. 2 sec. How much kinetic energy does it have?

Sample Problems • A 10 kg object is 26 m off the ground a. ) How much kinetic energy will it have if it falls to the ground? b. ) How much work will it do as it falls?

Another Sample Problem • An object moves 6 m in 12. 1 s and weighs 5. 6 N. How much work will the object do as it moves?

Energy Conservation – The fact that all three types of energy are interrelated means energy is conserved (the same as momentum) – The full statement of the conservation of energy is: “in a closed system, energy is neither created nor destroyed, it just changes form. ”

Notes NOW! • Just as momentum and energy are conserved, so is matter. The conservation of matter law states; “Matter is neither created or destroyed, it just changes form. ” • This means that matter can be converted to energy and back again. • Einstein in 1908 developed a formula to show that matter can be converted to energy – it is the most famous of Physics equations…

ANOTHER FORMULA E=mc² E=energy m=mass c=speed of light (3 x 10 m/s)

Lastly • A final formula shows conservation of energy: • E(T)=PE + KE +Q Q=Heat • E(T)=Total energy of the system • This formula shows that energy is not created or destroyed, but just changes form

SAMPLE…the final one. • How much energy is released when an atomic nucleus of mass 1000 kg is destroyed?