Regents Chemistry Topic 2 The Periodic Table and
Regents Chemistry Topic 2 The Periodic Table and Formulas / Equations
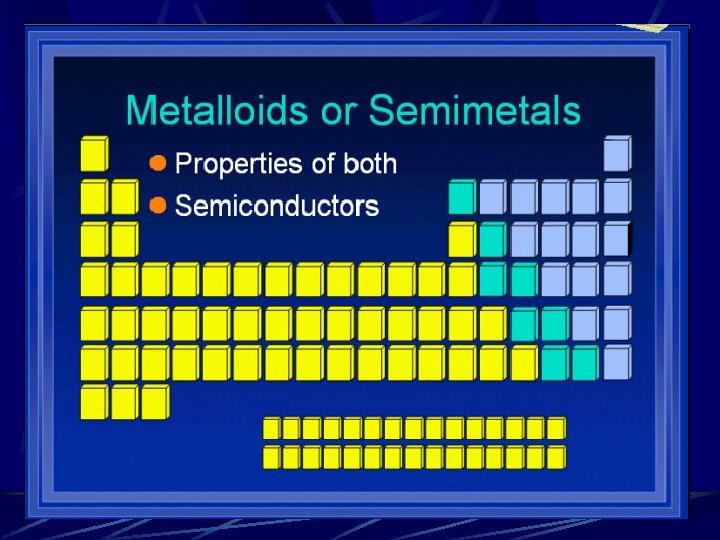
Regents Chemistry Introduction to the Table Groups and Families l Elements of Metals, Nonmetals and Metalloids l

Recap • The nucleus contains protons and neutrons Nucleus • Electrons surround the nucleus in a “cloud” • Atomic number is the number of protons • Atomic mass is the sum of protons and neutrons electrons

The Periodic Table The periodic table is arranged according to Atomic Number The first table, by Russian chemist Dmitri Mendeleev, was arranged by atomic mass, but this was not accurate Current arrangement shows many important trends. .
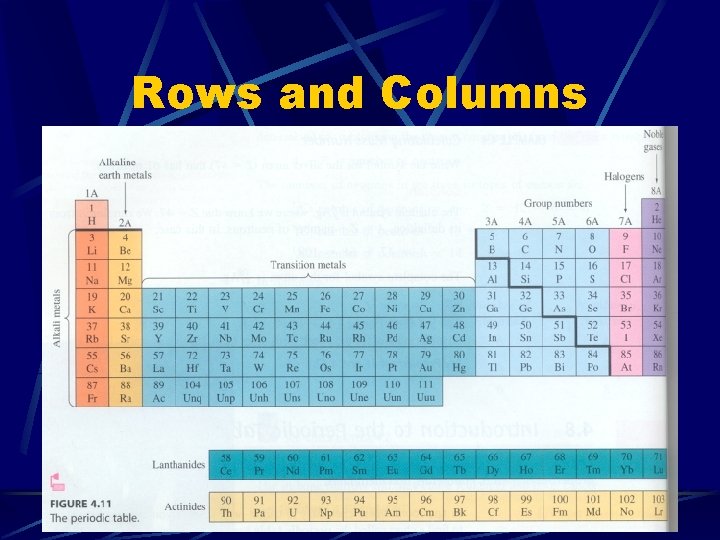
Rows and Columns
Divisions of the Periodic Table Metals Alkali metals l Alkaline earth metals l Transition Metals l Metalloids Nonmetals Halogens l Nobel gases l
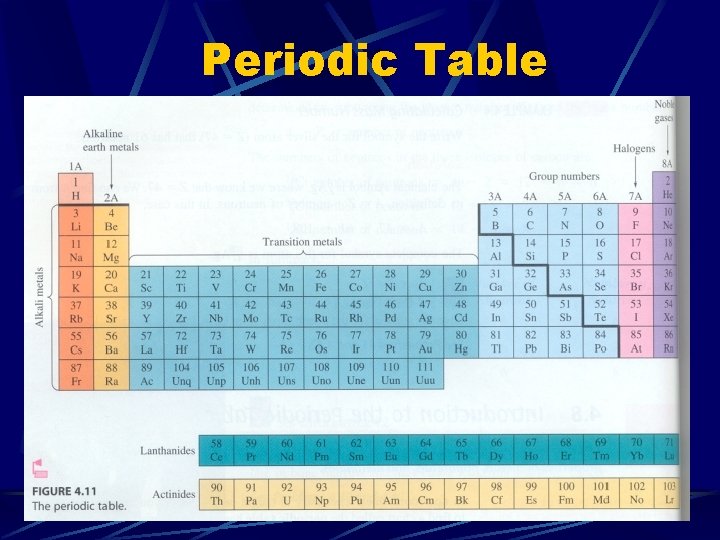
Periodic Table
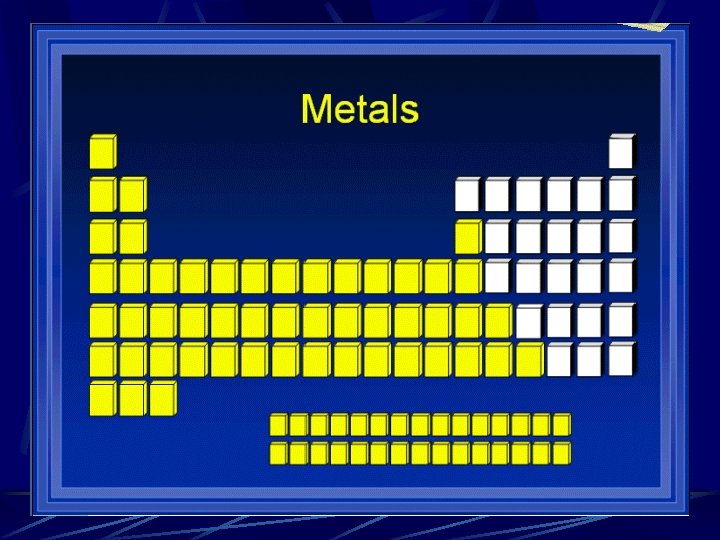
Physical Properties of Metals 1. Efficient conduction of heat and electricity 2. Malleability (they can be hammered into 3. thin sheets 4. 3. Ductility (they can be pulled into wires) 5. 4. A lustrous (shiny) appearance
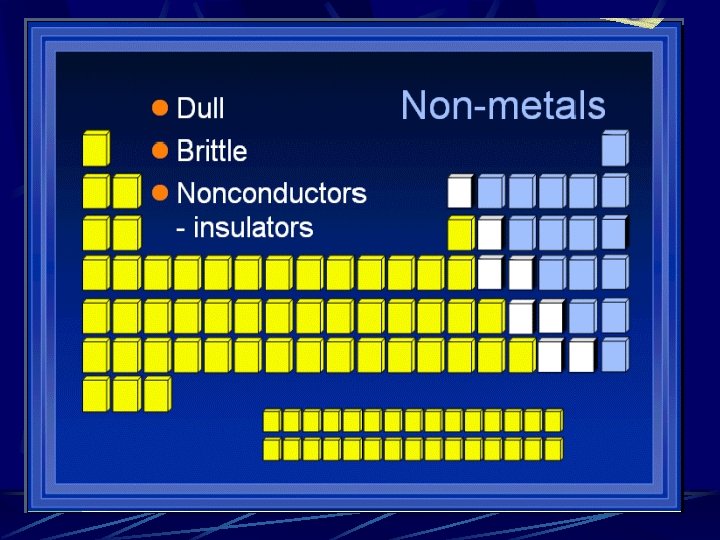

Natural States of Elements Most of the matter around us consists of mixtures Mixtures contain compounds Atoms of individual elements are not often found in nature in pure form Some exceptions: gold, platinum and silver l Also noble gases – do not combine readily l l Ex; Helium gas in underground deposits

Elements after Separation. . After we use a chemical process to separate the elements in a compound, we find the elements to be: Monoatomic atoms– only (1) atom of the element l Diatomic molecules– (2) atoms bonded together l
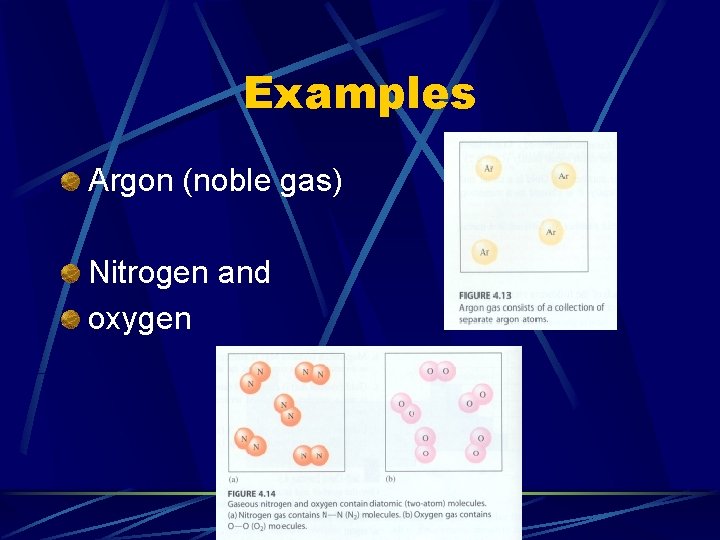
Examples Argon (noble gas) Nitrogen and oxygen
Natural Physical States Metals are solids at 25 C Noble gases are gas at room 25 C and are individual atoms Several others are gases and diatomic at 25 C – (H 2, N 2, O 2, F 2, Cl 2) Only two elements are liquids at 25 C l Bromine and Mercury

Elements can have different forms… Solid metals differ from solid non-metals In fact, different forms of the same element can occur l These are called allotropes Ex: Carbon Diamond (very hard) l Graphite (soft) l Buckministerfullerene (newly discovered) l End
Regents Chemistry Information on the Table Average Atomic Mass l Atomic Number l Isotopes l

Regents Chemistry Periodic Table Bingo

Regents Chemistry Ions and Oxidation States


**Change ending of parent name to -ide and add word - ion**
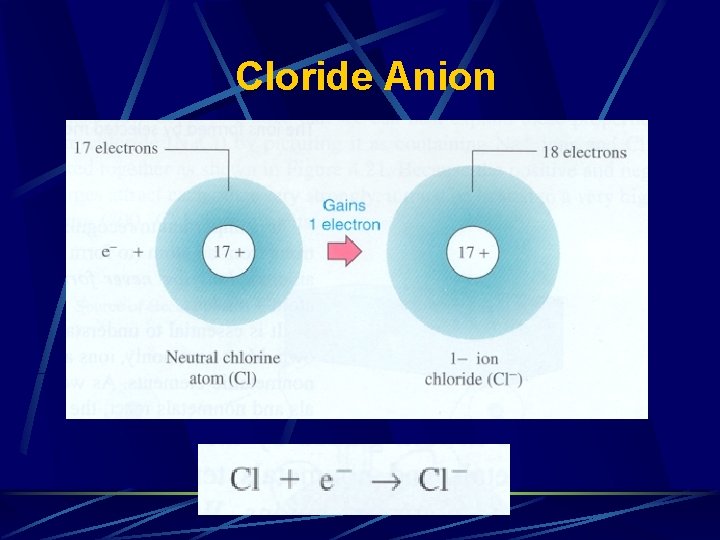
Cloride Anion

***Keep parent name and add word - ion***
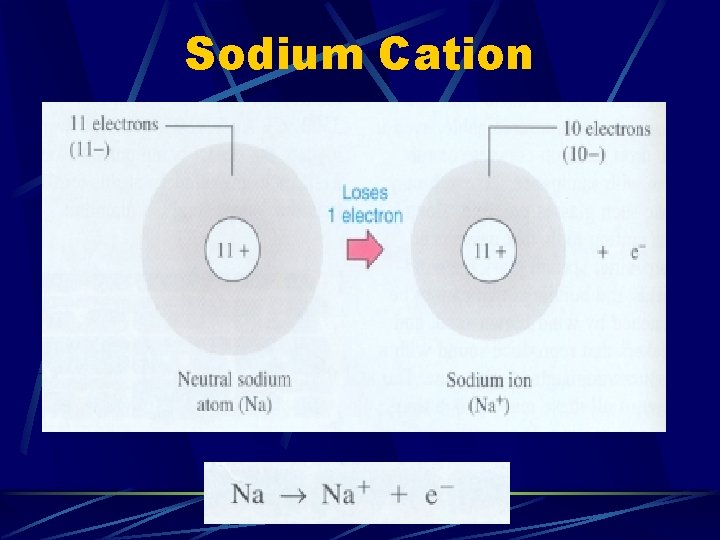
Sodium Cation

Ionic charges from Periodic Table See pg. 112
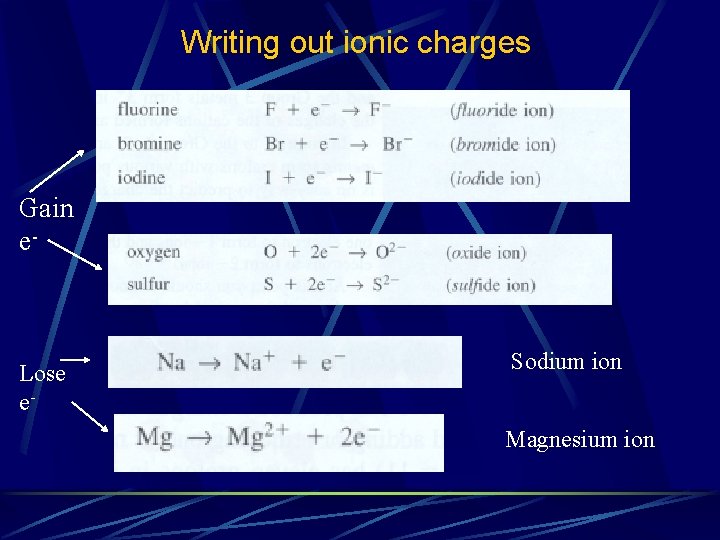
Writing out ionic charges Gain e- Lose e- Sodium ion Magnesium ion

Ionic Compounds Combination of cation and anion l Write cation first and anion second When combining, we must consider electrical charge l Ions combine in such a way to make a zero net charge Total charge of cations + Total charge of anion = Zero net charge
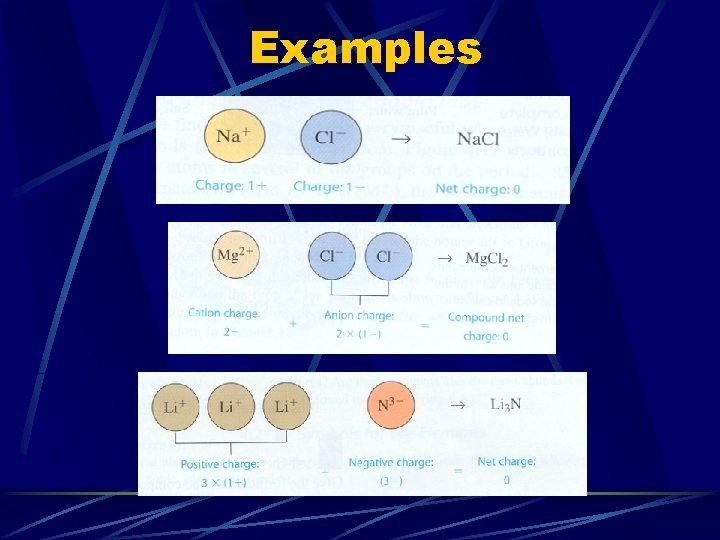
Examples
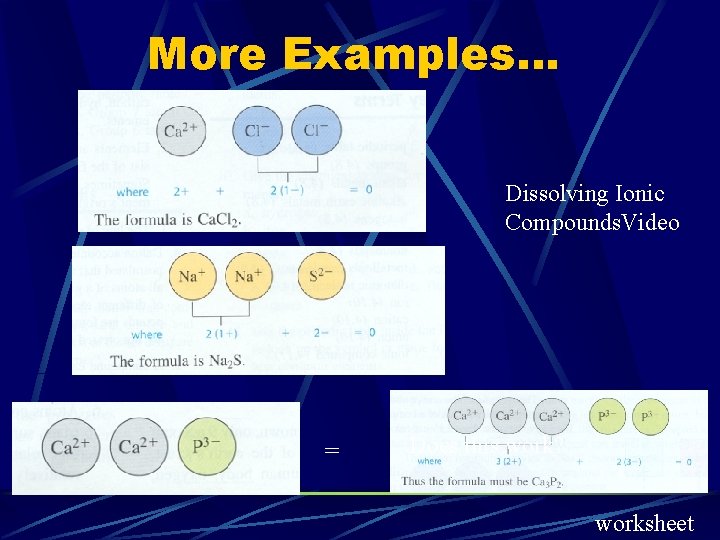
More Examples. . . Dissolving Ionic Compounds. Video = Does this work? worksheet

Regents Chemistry Chemical Nomenclature

Naming Compounds Common names were originally developed to name compounds l Ex: Epsom salts, milk of magnesia, gypsum and laughing gas Too many common names. . a system had to be developed!

Naming Compounds Binary compounds – compounds that are composed of two elements We will examine two classes of binary compounds 1. Compounds that contain a metal and a nonmetal l 2. Compounds that contain two nonmetals l

Naming Binary Ionic Compounds Binary ionic compounds result when a metal combines with a nonmetal The metal loses electrons as the nonmetal gains electrons The result is a positive cation (the metal) and a negative anion (the nonmetal) In naming ionic compounds, we simply name the ions

Naming Binary Ionic Compounds We will learn how to name two types of ionic compounds (polyatomic ion naming will come later) l Type I compounds – The metal is present in only one type of cation - look at periodic table! l l Ex: Na+, K+, Ca 2+, Al 3+ Type II compounds – The metal present can form two (or more) cations that have different charges - look at periodic table! l Ex: Cr 2+, Cr 3+, Cu 2+
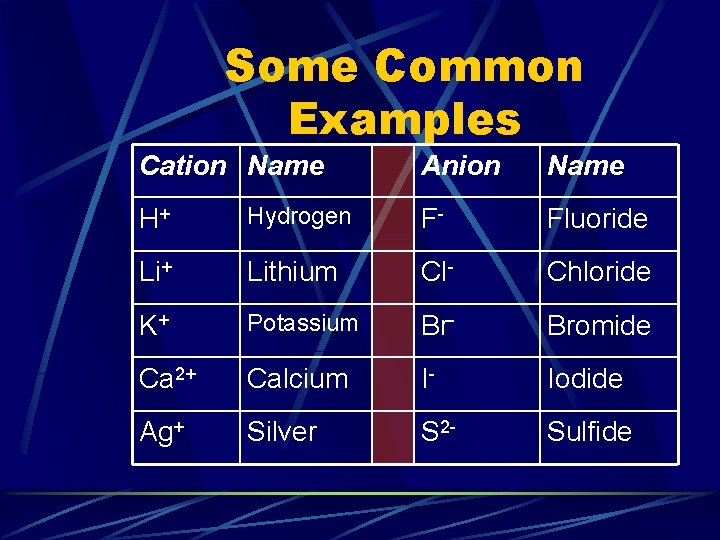
Some Common Examples Cation Name Anion Name H+ Hydrogen F- Fluoride Li+ Lithium Cl- Chloride K+ Potassium Br- Bromide Ca 2+ Calcium I- Iodide Ag+ Silver S 2 - Sulfide

Naming Rules for Type I Ionic 1. The cation is always named first and the anion second 2. A simple cation (obtained from a single atom) takes its name from the name of the element. l Ex: Na+ Sodium ion 3. A simple anion (obtained from a single atom) is named by taking the first part of the elemental name and adding – ide l Ex: F- Fluoride ion

Examples Name the following compounds CLICK TO REVEAL ANSWERS • Na. Cl sodium chloride • KI potassium iodide • Ca. S calcium sulfide STOP worksheet

Regents Chemistry Naming Type II Compounds
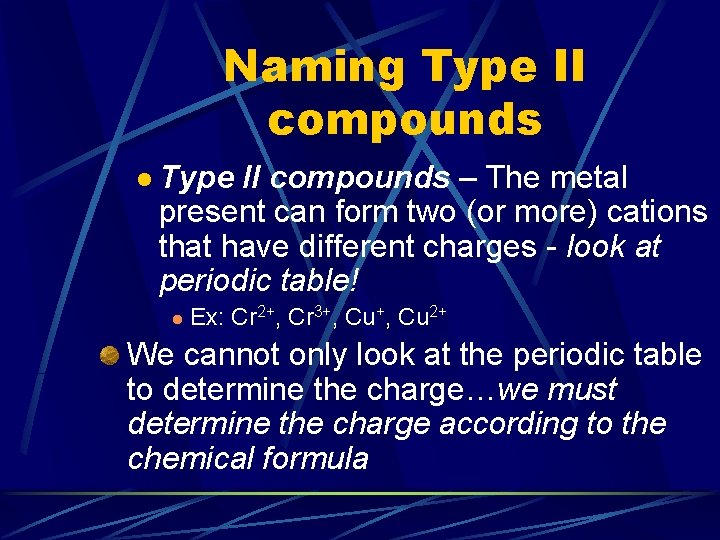
Naming Type II compounds l Type II compounds – The metal present can form two (or more) cations that have different charges - look at periodic table! l Ex: Cr 2+, Cr 3+, Cu 2+ We cannot only look at the periodic table to determine the charge…we must determine the charge according to the chemical formula

Determing the correct charge All compounds must be electrically neutral. . so we use the charge of the anion to determine the charge of the cation…and multiply the charges by the number of atoms to determine the overall net charge

Example Cu. Cl Cl comes in as Cl- : -1 x 1 Cl ion = -1 Cu must come in as a +1 : +1 x 1 Cu ion = +1 -1 + +1 = 0 , the charges balance Copper (I) Chloride
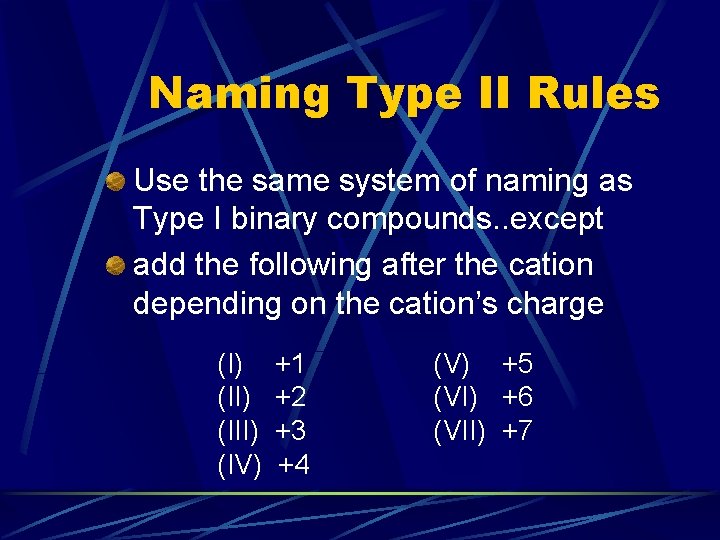
Naming Type II Rules Use the same system of naming as Type I binary compounds. . except add the following after the cation depending on the cation’s charge (I) (III) (IV) +1 +2 +3 +4 (V) +5 (VI) +6 (VII) +7
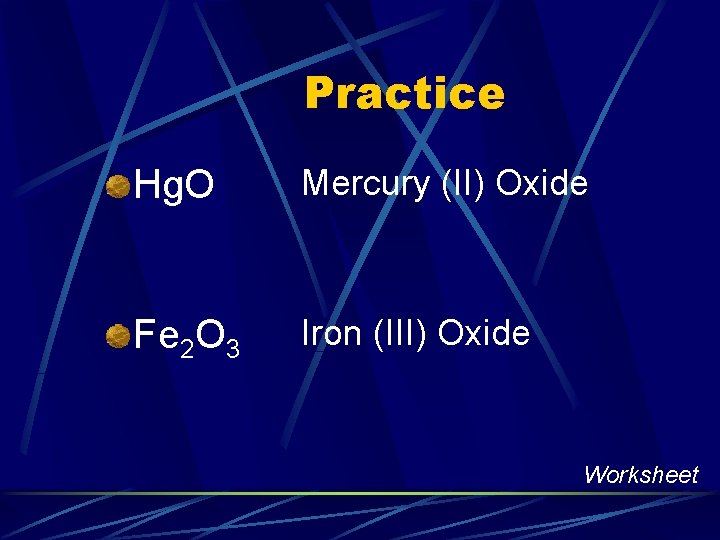
Practice Hg. O Mercury (II) Oxide Fe 2 O 3 Iron (III) Oxide Worksheet
Regents Chemistry Naming Type III Binary Compounds Non-metal to non-metal

Type III Binary Compounds - are compounds that contain only nonmetals participating in covalent bonds (sharing of electrons)

Rules for Naming Type III 1. The first element in the formula is named first, and the full element name is used 2. The second element is named as though it were an anion (-ide ending) 3. Prefixes are used to denote the numbers of atoms present. 4. The prefix mono is never used for naming the first element
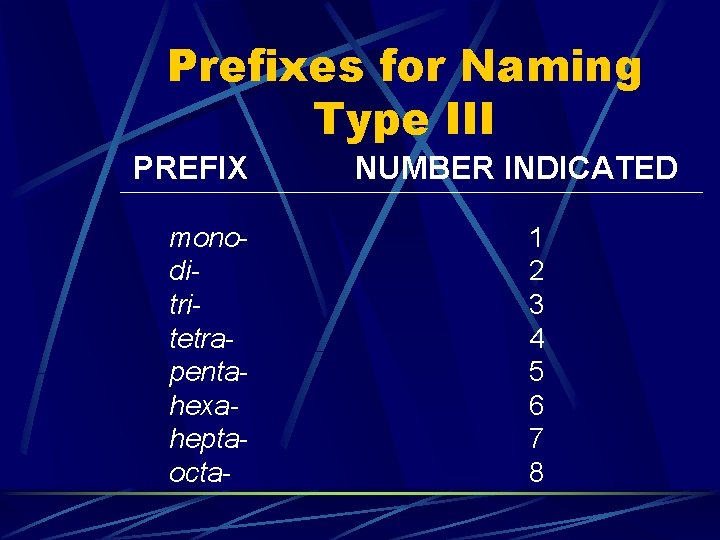
Prefixes for Naming Type III PREFIX monoditritetrapentahexaheptaocta- NUMBER INDICATED 1 2 3 4 5 6 7 8

Practice boron trifluoride BF 3 nitrogen monoxide NO N 2 O 5 dinitrogen pentoxide carbon tetrachloride CCl 4 worksheet
Regents Chemistry l Polyatomic Ion Compound Nomenclature

What’s a polyatomic ion? A polyatomic ion consists of two or more elements bonded together that posess an overall net charge that can be used to form an ionic bond with a metal cation We looked at some of these! SO 42 -

Polyatomic Ion Intro… Polyatomic ions have specific names… You must be able to recognize polyatomic ions in chemical formulas and chemical names You reference tables can help! l See the table on the front page

Naming Polyatomic Ions… Some polyatomic ions have general names that are made from modification of the names of the elements involved l Ex: NH 4+ Ammonium Ion CN- Cyanide Ion

Naming Oxyanions are polyatomic ions (anions) that contain atoms of an element and different numbers of oxygen atoms When there are two members in such a series, the anion with the lesser number of oxygen is given the ending –ite and the larger number ends in –ate SO 3 2 - Sulfite Ion SO 42 - Sulfate Ion

Naming Oxyanions cont… If there are more that two in the series, we use the prefix hypo for the member with the fewest oxygen and per for the one with the most oxygen Cl. O- hypochlorite ion Cl. O 2 - chlorite ion Cl. O 3 - chlorate ion Cl. O 4 - perchlorate ion
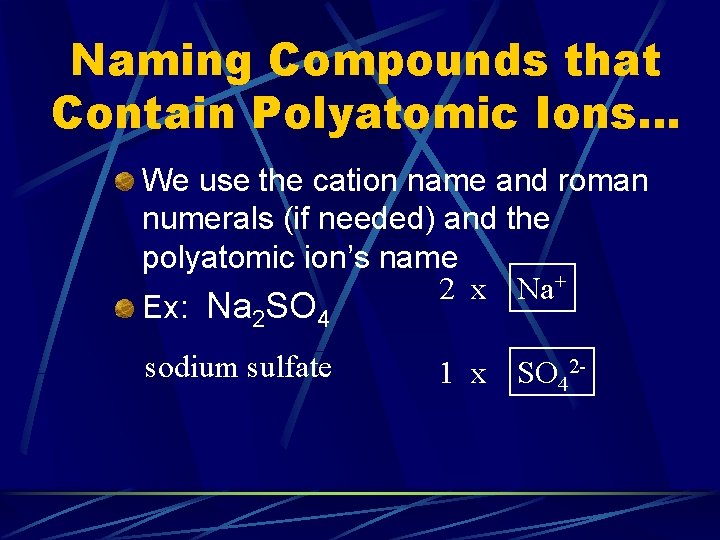
Naming Compounds that Contain Polyatomic Ions… We use the cation name and roman numerals (if needed) and the polyatomic ion’s name 2 x Na+ Ex: Na 2 SO 4 sodium sulfate 1 x SO 42 -

Examples Fe(NO 3)3 Mn(OH)2 Cu. SO 4 iron (III) nitrate manganese (II) hydroxide copper (II) sulfate
Regents Chemistry Revisiting the Periodic Table l Trends of the Table

Properties of Elements Trends to be familiar with: Ionization Energy l Atomic Radii l Ionic Radii l Electronegativity l Reactivity of Elements l

Summary of Trends Ionization Energy – increases from left to right and up the columns Atomic Radii – increases from right to left and down columns Ionic Radii – depends on if the atom looses or gains electrons Electronegativity – increases from left to right and up the columns Reactivity – Groups 1, 2 and 17 along with oxygen are most reactive

Families on the Table 3 – day website project: see handout
- Slides: 60