Refrigeration Introduction to Food Engineering Ice Box Ice

Refrigeration Introduction to Food Engineering
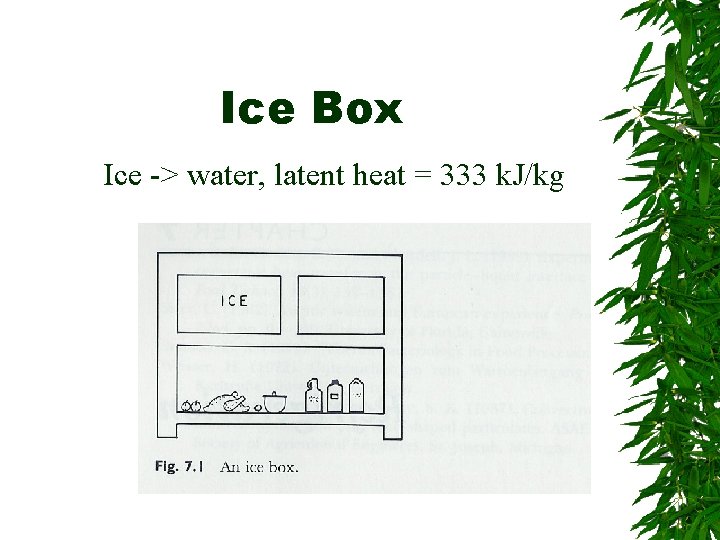
Ice Box Ice -> water, latent heat = 333 k. J/kg
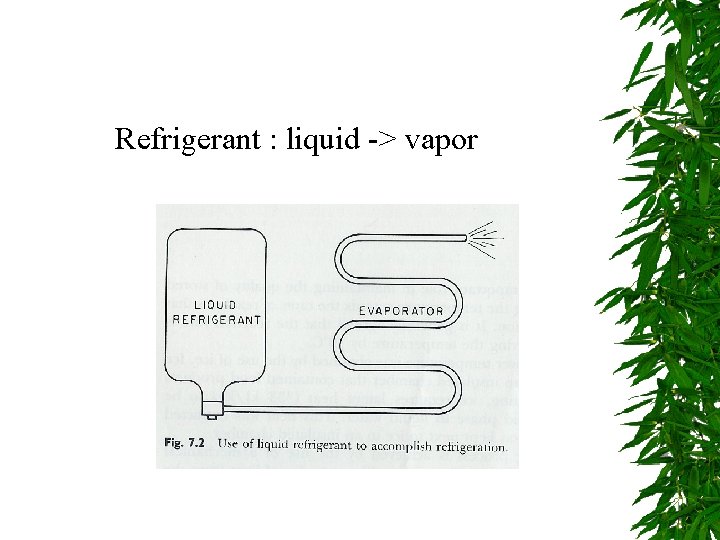
Refrigerant : liquid -> vapor
Selection of Refrigerant 1. Latent heat of vaporization – High value – Small amount needed per unit time 2. Condensing pressure – High pressure needs expenses on heavy construction of condenser & piping 3. Freezing temperature – Should be below evaporator temperature
Selection. . . 4. Critical temperature – Temperature that refrigerant vapor cannot be liquefied, should be high 5. Toxicity, must be non-toxic 6. Flammability – nonflammable 7. Corrosiveness – noncorrosive 8. Chemical stability - stable

Selection. . . 9. Detection of leaks – easy to detect 10. Cost 11. Environmental impact
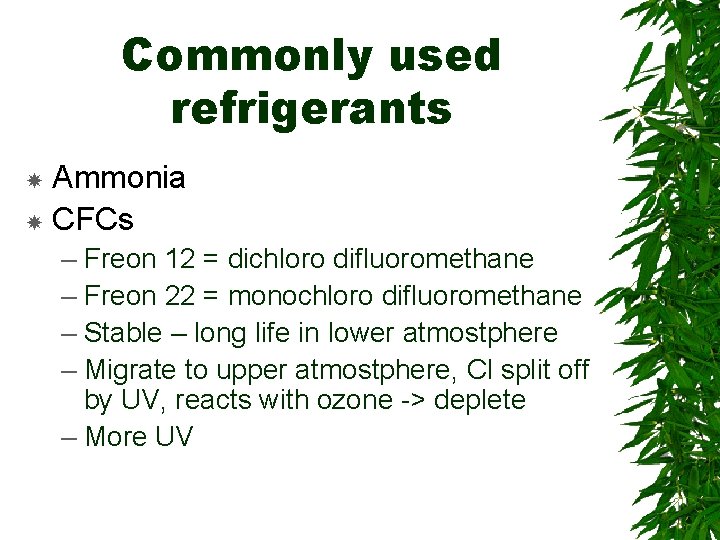
Commonly used refrigerants Ammonia CFCs – Freon 12 = dichloro difluoromethane – Freon 22 = monochloro difluoromethane – Stable – long life in lower atmostphere – Migrate to upper atmostphere, Cl split off by UV, reacts with ozone -> deplete – More UV

Alternatives HCFs – Less stable – Hydrofluorocarbons – hydrochlorofluorocarbons
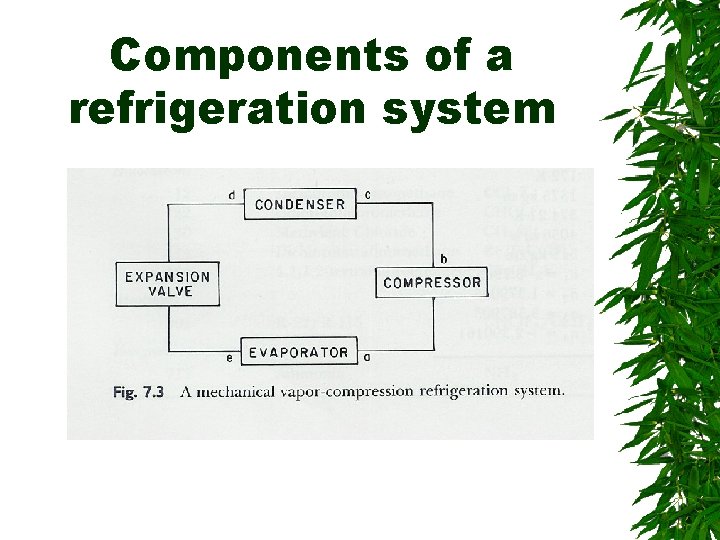
Components of a refrigeration system
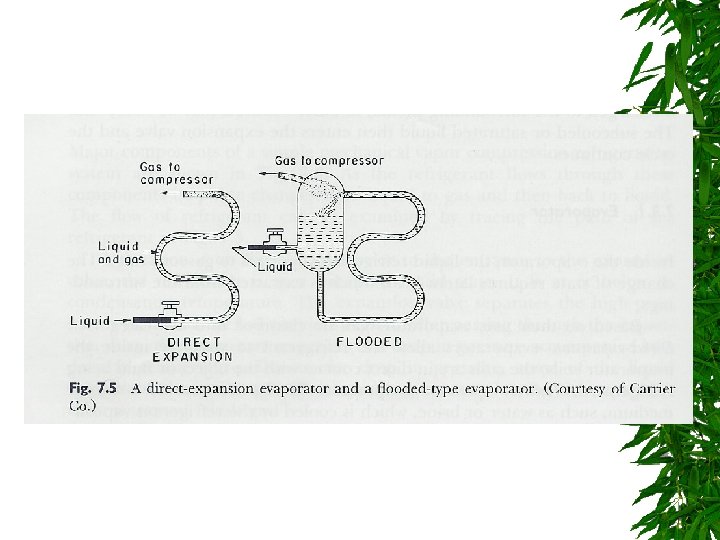
Evaporator
Compressor
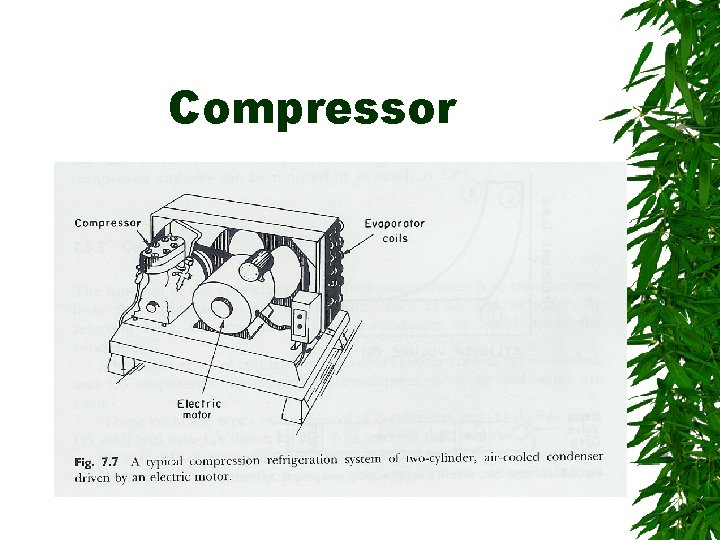
Compressor
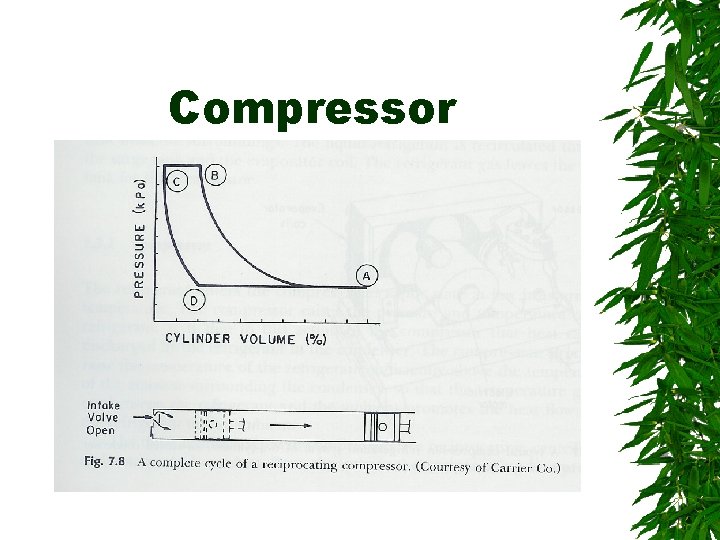
Compressor
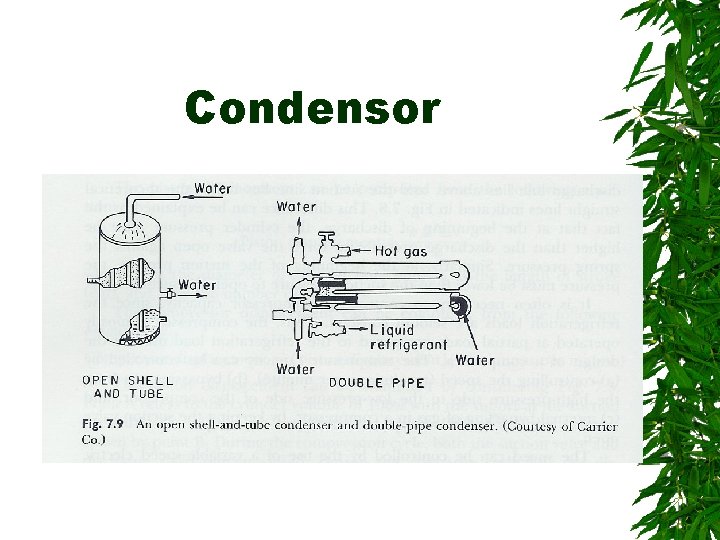
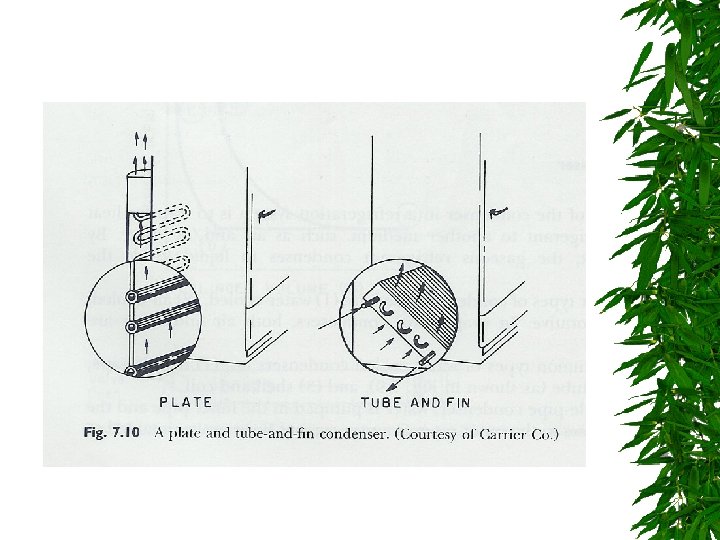
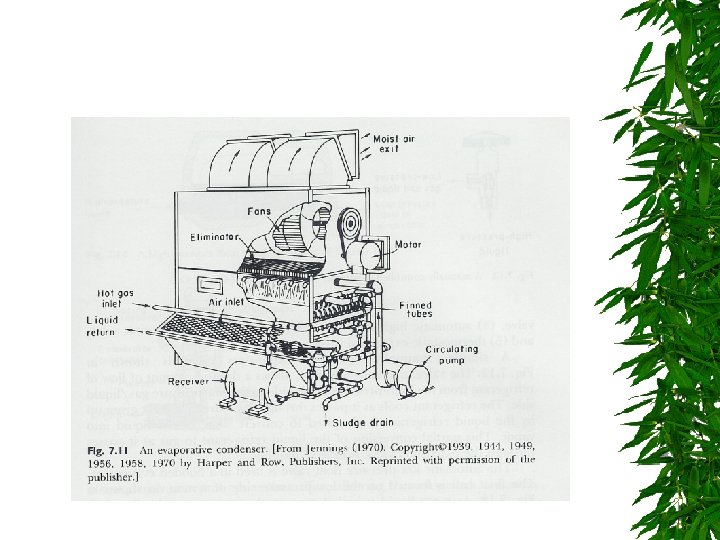
Condensor
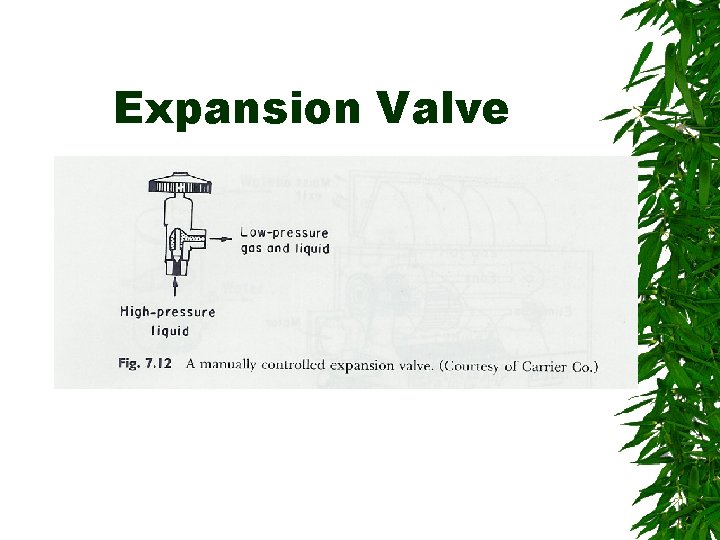
Expansion Valve
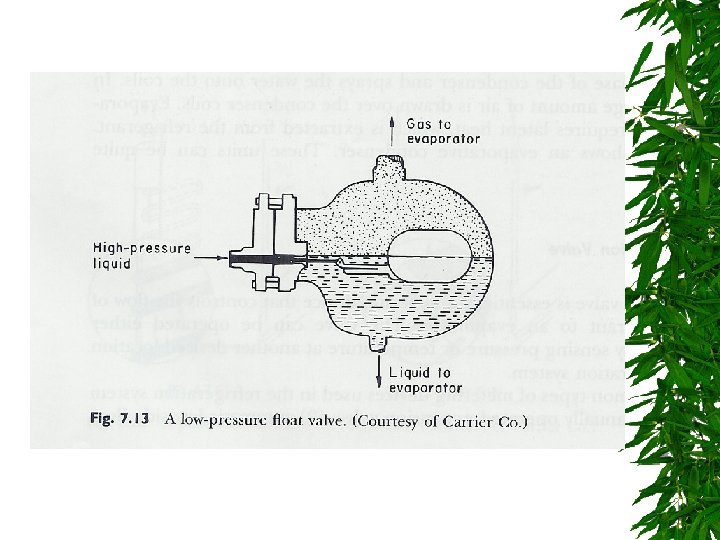
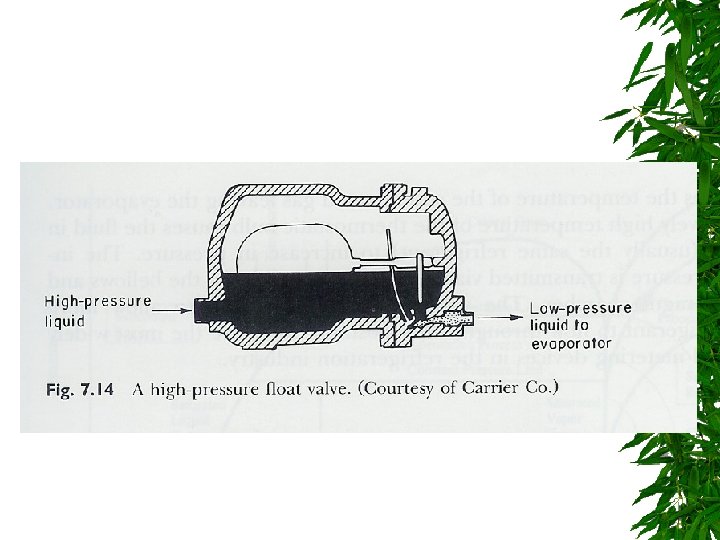
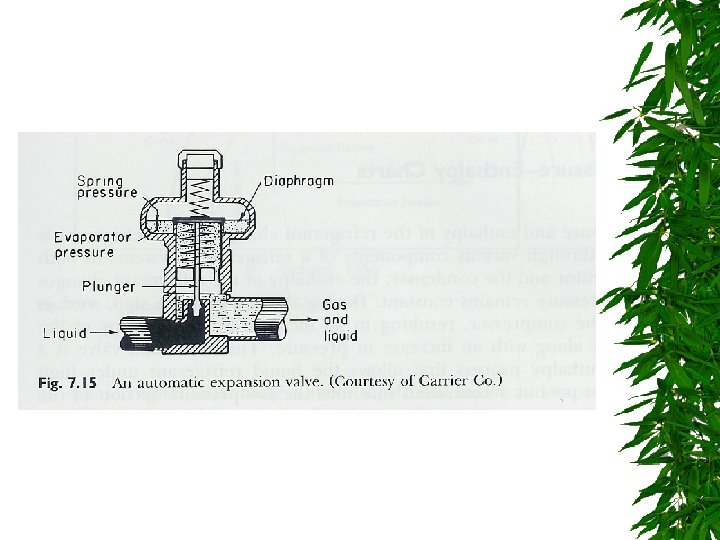
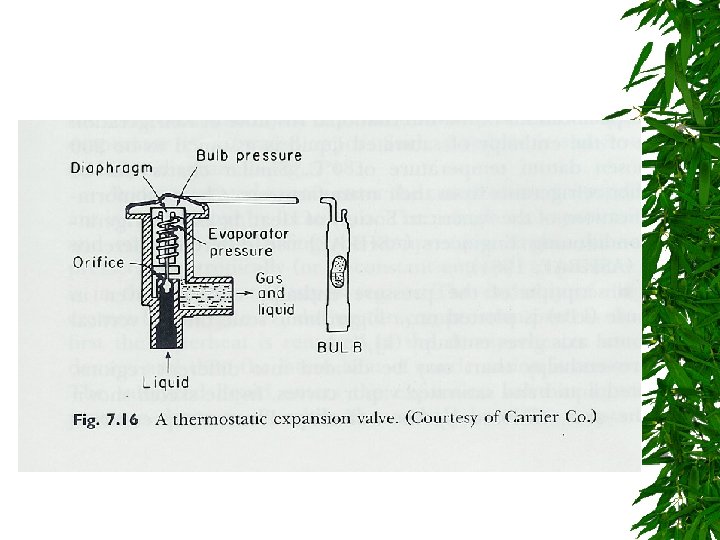
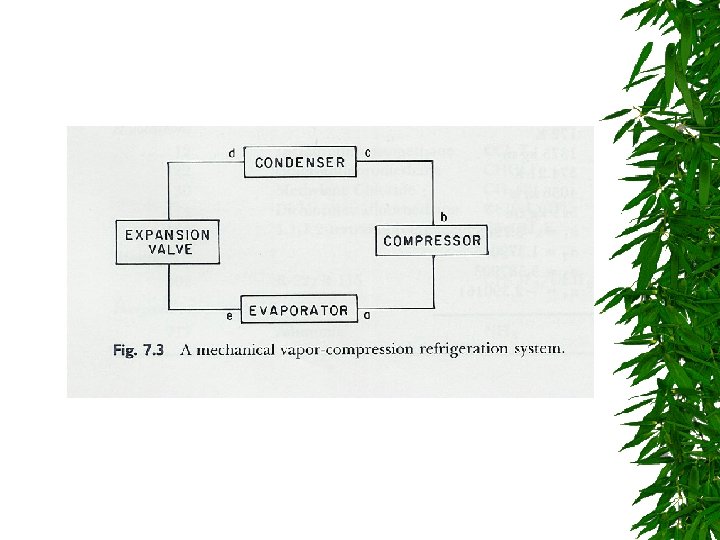

Changes d saturated liquid condensation temp Passing through expansion valve – Pressure & temp drop – Some liquid -> gas Liquid/gas mixture enters evaporator coils at e – Completely vaporize, -> saturated vapor (gain additional heat)

Changes. . . Vapors enter compressor – Compressed -> high pressure, temp increase – Superheated refrigerant Superheated vapor cooled by air or water in condenser – Saturated liquid
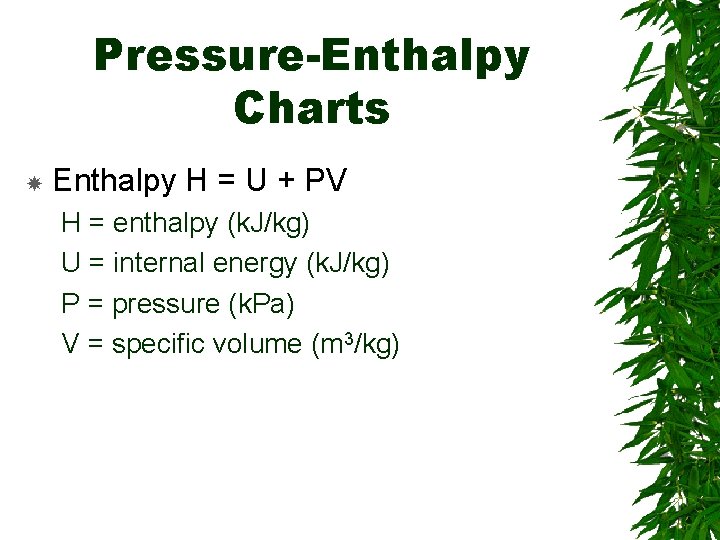
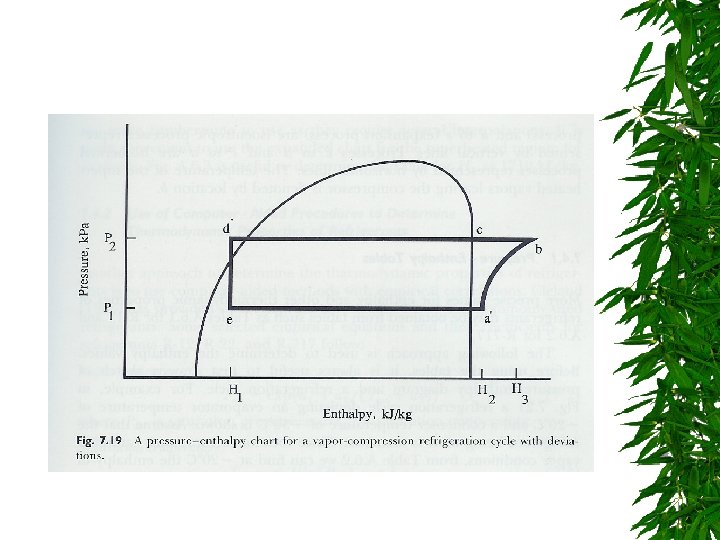
Pressure-Enthalpy Charts Enthalpy H = U + PV H = enthalpy (k. J/kg) U = internal energy (k. J/kg) P = pressure (k. Pa) V = specific volume (m 3/kg)

Evaporator & Condenser – Enthalpy change, pressure constant Compression : work done – Increase enthalpy, increase pressure Expansion valve – Constant enthalpy – Flow from high P -> low P
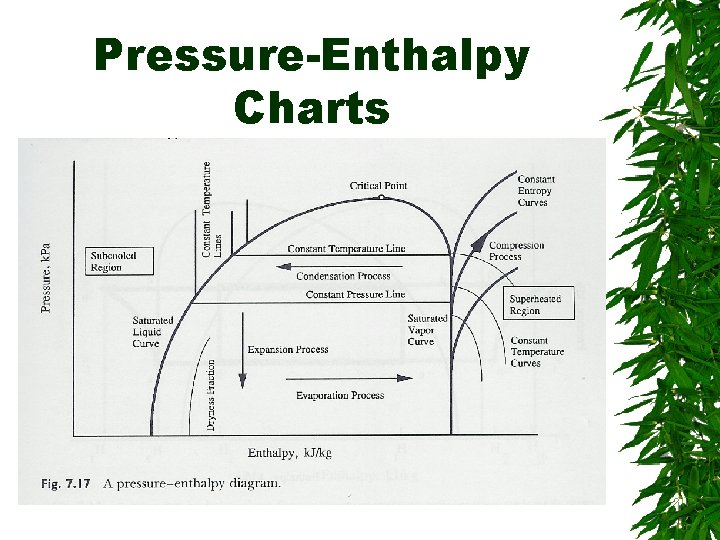
Pressure-Enthalpy Charts
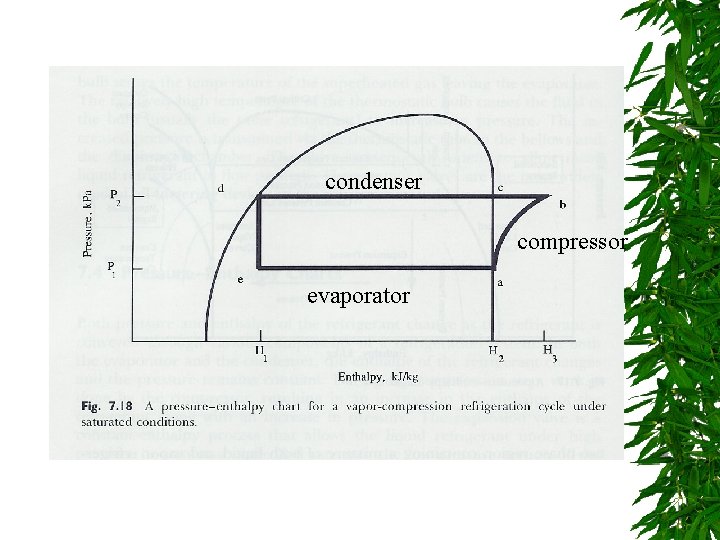
condenser compressor evaporator

Cooling Load Rate of heat energy removal from a given space or object to lower temp. to a desired level One ton of refrigeration = latent heat of fusion of 1 ton of ice = 288, 000 Btu/24 hr = 303, 852 k. J/24 hr = 3. 5168 k. W

Cooling load calculation must consider heat of respiration, walls, floor, doors, etc.

Example Calculate cooling load caused by heat of evolution of 2000 kg cabbage stored at 5 °C. Given heat of evolution of cabbage at 5 °C = 28 – 63 W/Mg Total heat evolution (2000 kg)(63 W/Mg)(1 Mg/1000 kg) = 126 W
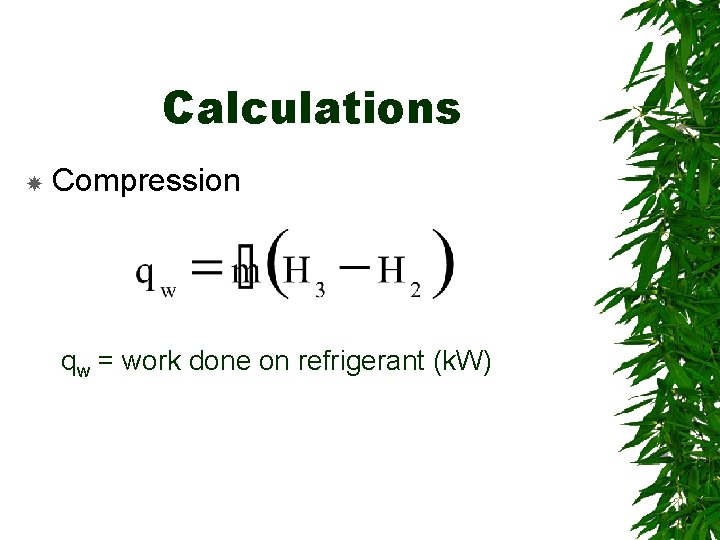
Calculations Compression qw = work done on refrigerant (k. W)
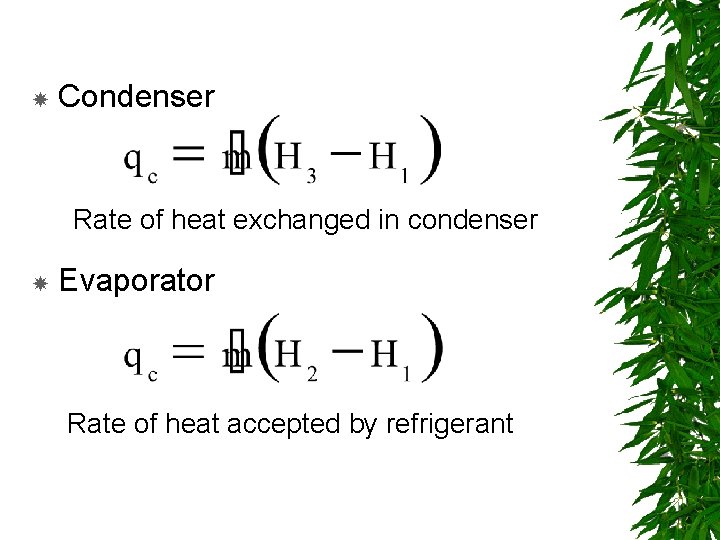
Condenser Rate of heat exchanged in condenser Evaporator Rate of heat accepted by refrigerant
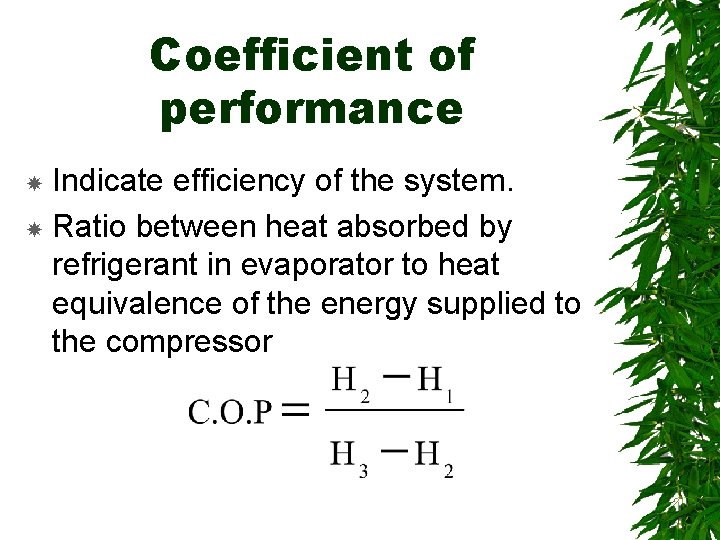
Coefficient of performance Indicate efficiency of the system. Ratio between heat absorbed by refrigerant in evaporator to heat equivalence of the energy supplied to the compressor
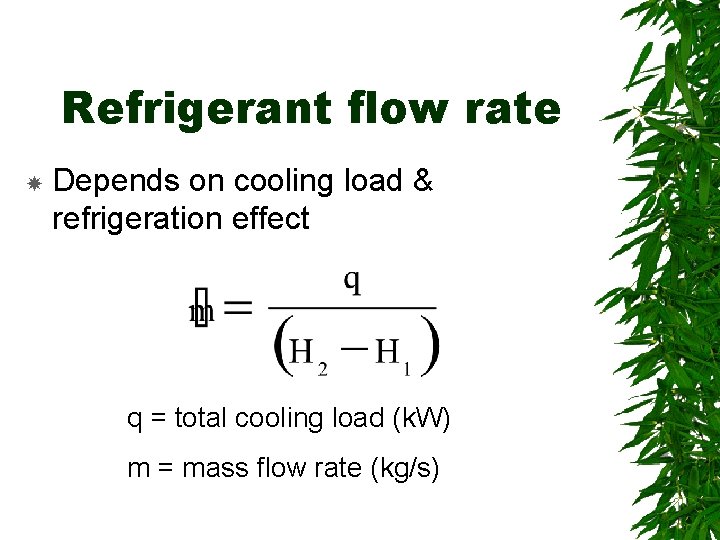
Refrigerant flow rate Depends on cooling load & refrigeration effect q = total cooling load (k. W) m = mass flow rate (kg/s)
Example Cold storage room (2 °C) uses Freon 12 as refrigerant. Evaporator temp = -5 °C, Condenser temp = 40 °C, refrigeration load = 20 tons, calculate m, compressor power requirement and C. O. P. Assume saturated conditions and compressor efficiency 85 %.
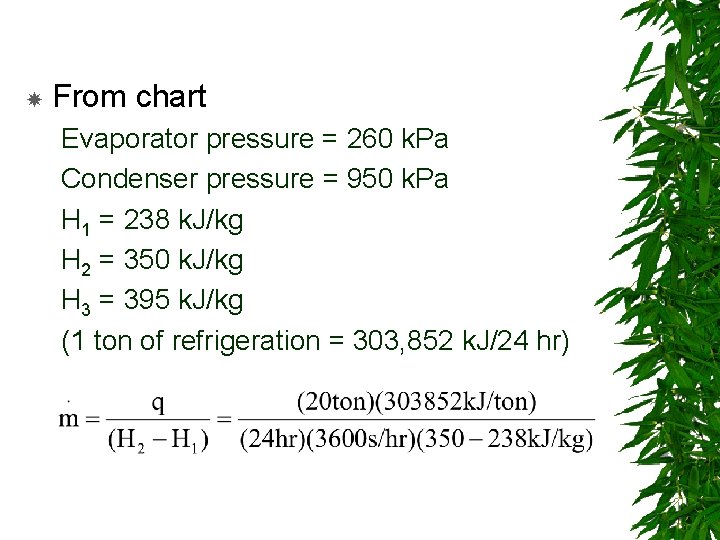
From chart Evaporator pressure = 260 k. Pa Condenser pressure = 950 k. Pa H 1 = 238 k. J/kg H 2 = 350 k. J/kg H 3 = 395 k. J/kg (1 ton of refrigeration = 303, 852 k. J/24 hr)
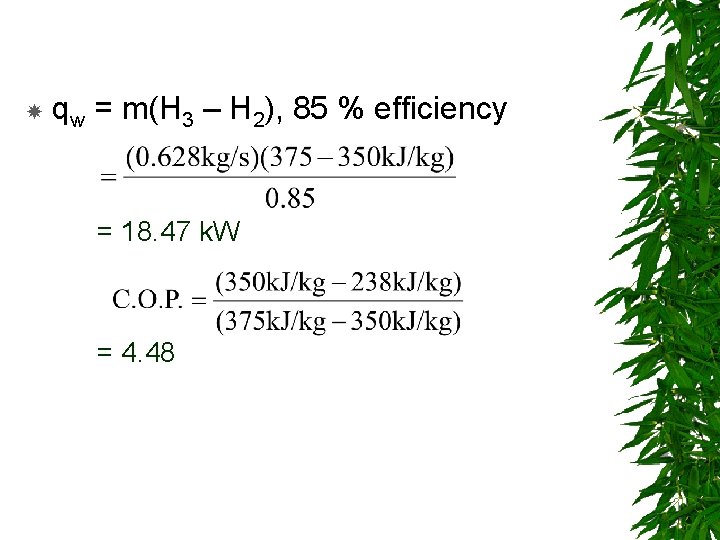
qw = m(H 3 – H 2), 85 % efficiency = 18. 47 k. W = 4. 48

Assume vapors leave evaporator 10 °C super-heated, liquid from condenser is subcooled 15 °C. – m = 0. 54 kg/s – qw = 15. 9 k. W – C. O. P. = 5. 2 Slower m, less power, higher C. O. P.
- Slides: 42