Reduction of Aldehydes and Ketones 19 8 Reduction

- Slides: 25

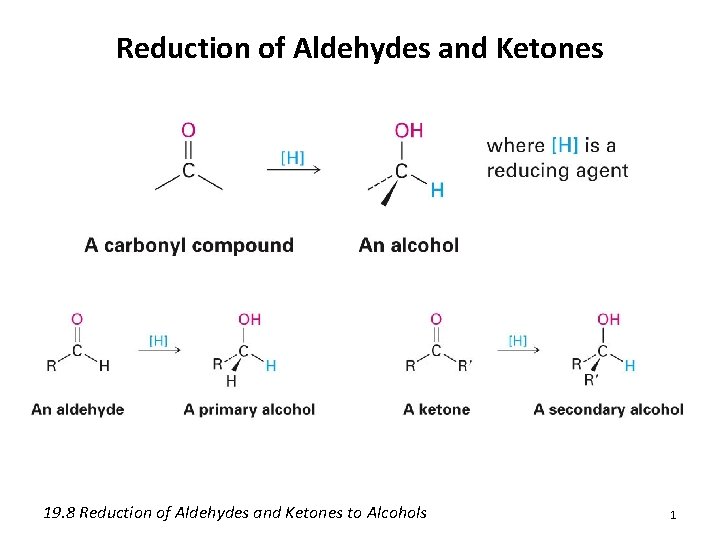
Reduction of Aldehydes and Ketones 19. 8 Reduction of Aldehydes and Ketones to Alcohols 1

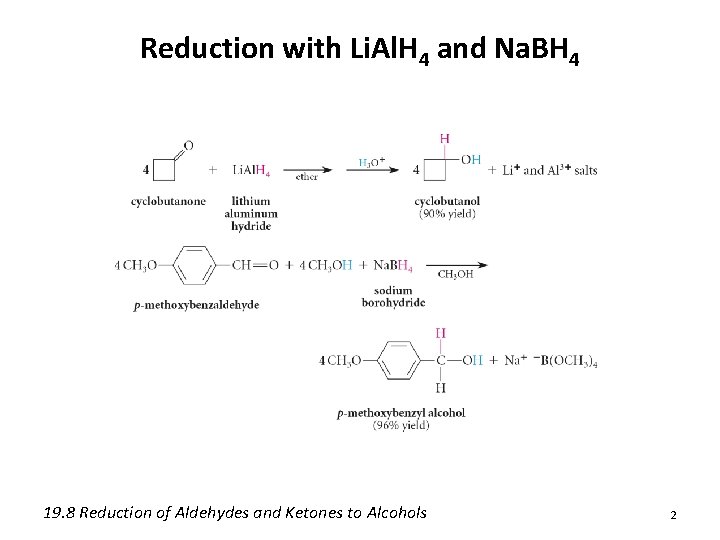
Reduction with Li. Al. H 4 and Na. BH 4 19. 8 Reduction of Aldehydes and Ketones to Alcohols 2

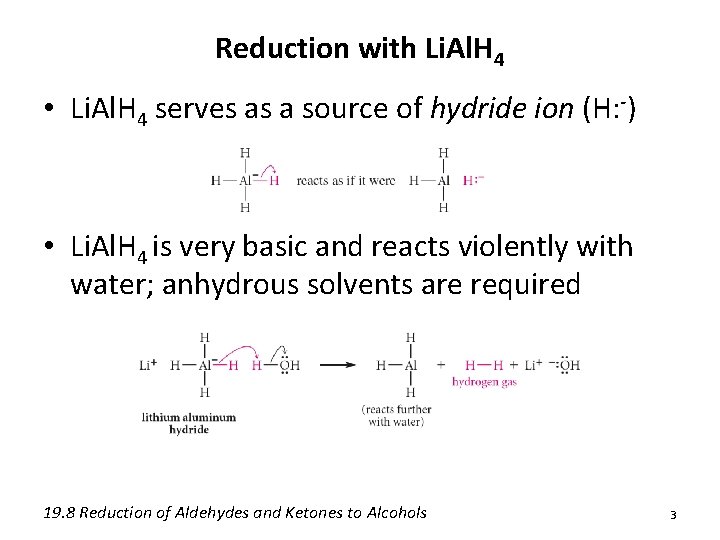
Reduction with Li. Al. H 4 • Li. Al. H 4 serves as a source of hydride ion (H: -) • Li. Al. H 4 is very basic and reacts violently with water; anhydrous solvents are required 19. 8 Reduction of Aldehydes and Ketones to Alcohols 3

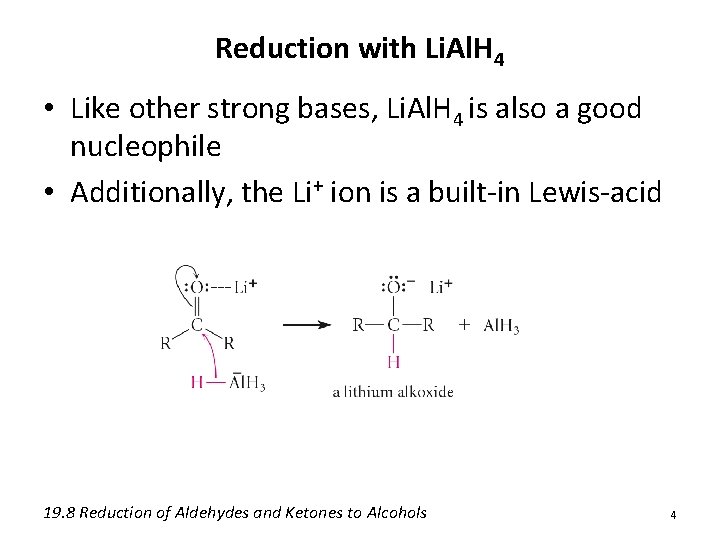
Reduction with Li. Al. H 4 • Like other strong bases, Li. Al. H 4 is also a good nucleophile • Additionally, the Li+ ion is a built-in Lewis-acid 19. 8 Reduction of Aldehydes and Ketones to Alcohols 4

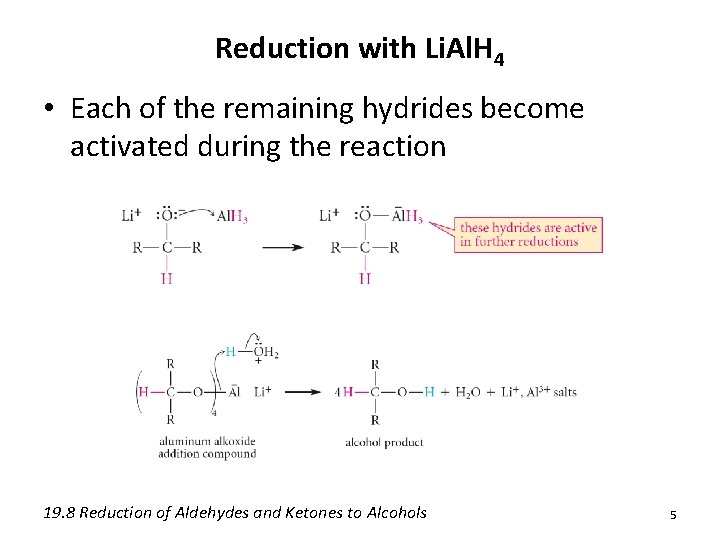
Reduction with Li. Al. H 4 • Each of the remaining hydrides become activated during the reaction 19. 8 Reduction of Aldehydes and Ketones to Alcohols 5

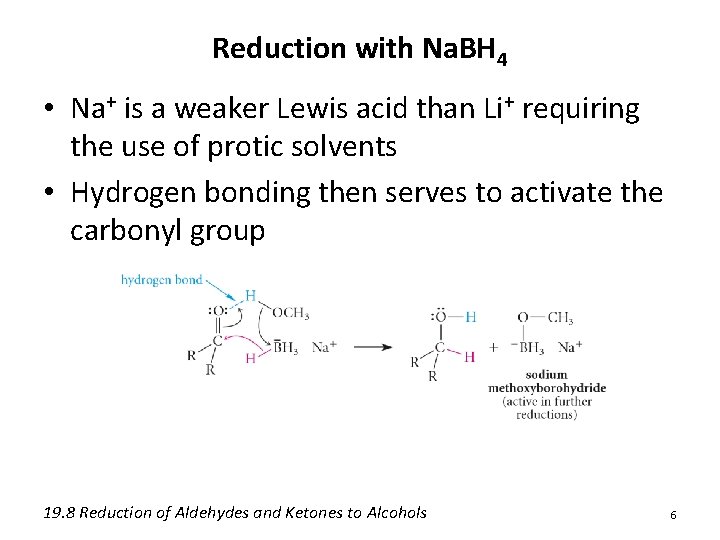
Reduction with Na. BH 4 • Na+ is a weaker Lewis acid than Li+ requiring the use of protic solvents • Hydrogen bonding then serves to activate the carbonyl group 19. 8 Reduction of Aldehydes and Ketones to Alcohols 6

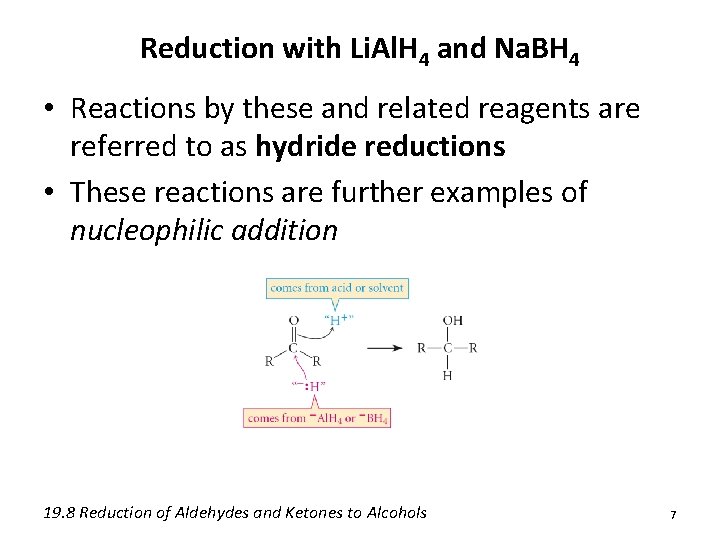
Reduction with Li. Al. H 4 and Na. BH 4 • Reactions by these and related reagents are referred to as hydride reductions • These reactions are further examples of nucleophilic addition 19. 8 Reduction of Aldehydes and Ketones to Alcohols 7

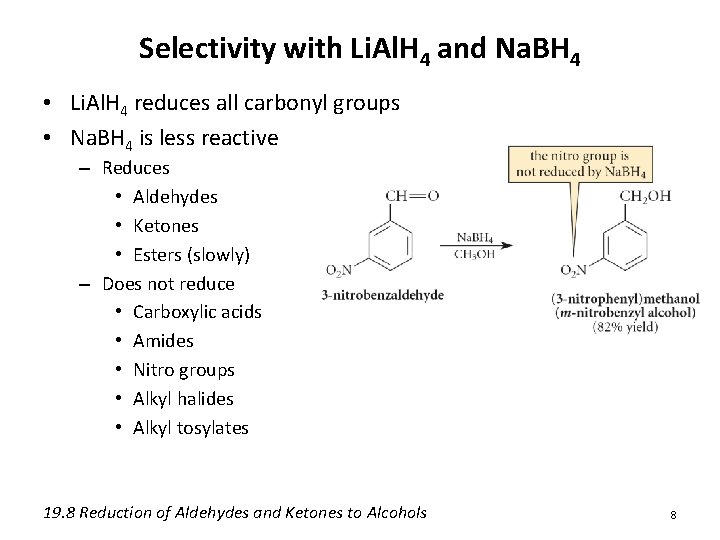
Selectivity with Li. Al. H 4 and Na. BH 4 • Li. Al. H 4 reduces all carbonyl groups • Na. BH 4 is less reactive – Reduces • Aldehydes • Ketones • Esters (slowly) – Does not reduce • Carboxylic acids • Amides • Nitro groups • Alkyl halides • Alkyl tosylates 19. 8 Reduction of Aldehydes and Ketones to Alcohols 8

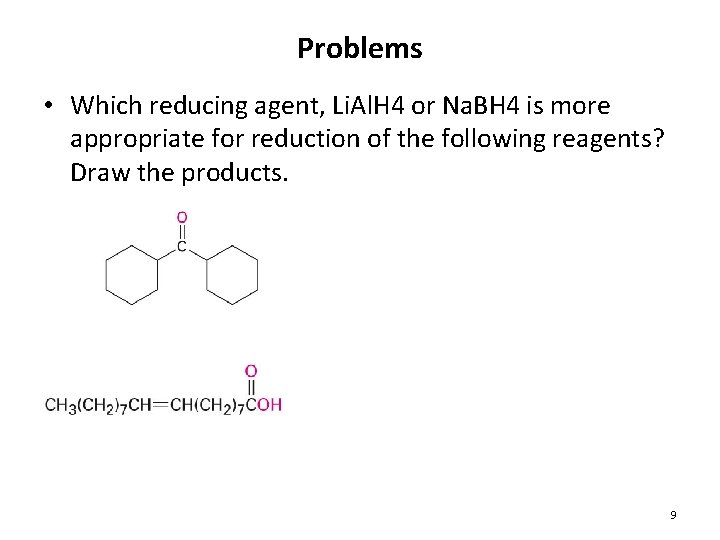
Problems • Which reducing agent, Li. Al. H 4 or Na. BH 4 is more appropriate for reduction of the following reagents? Draw the products. 9

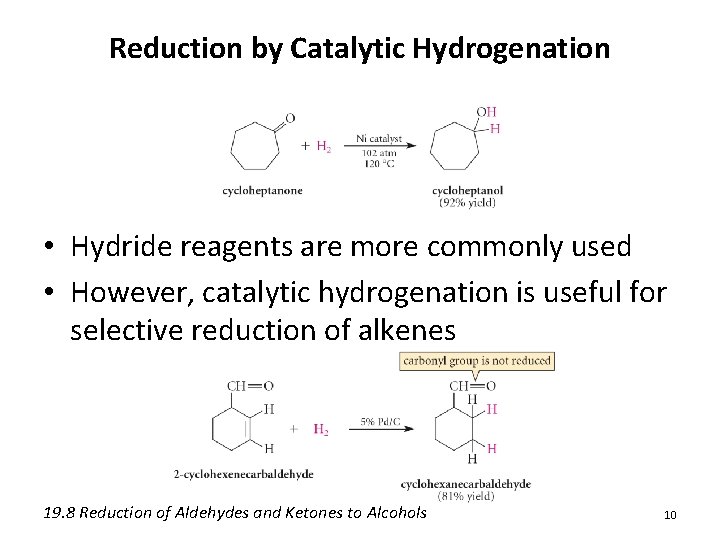
Reduction by Catalytic Hydrogenation • Hydride reagents are more commonly used • However, catalytic hydrogenation is useful for selective reduction of alkenes 19. 8 Reduction of Aldehydes and Ketones to Alcohols 10

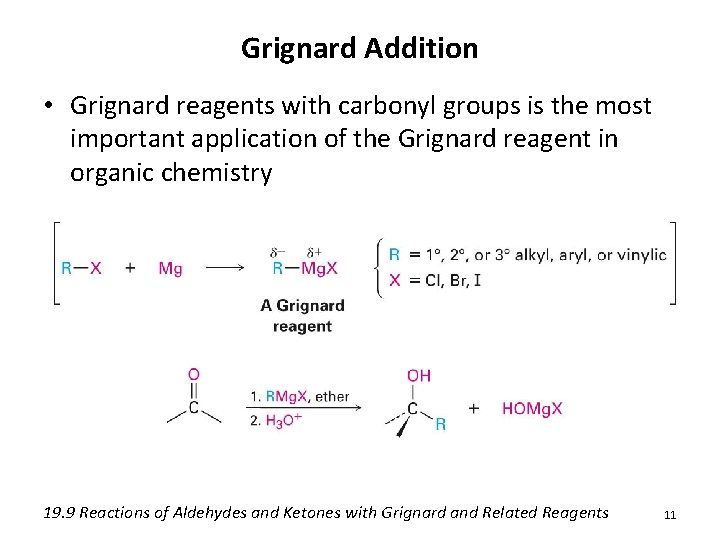
Grignard Addition • Grignard reagents with carbonyl groups is the most important application of the Grignard reagent in organic chemistry 19. 9 Reactions of Aldehydes and Ketones with Grignard and Related Reagents 11

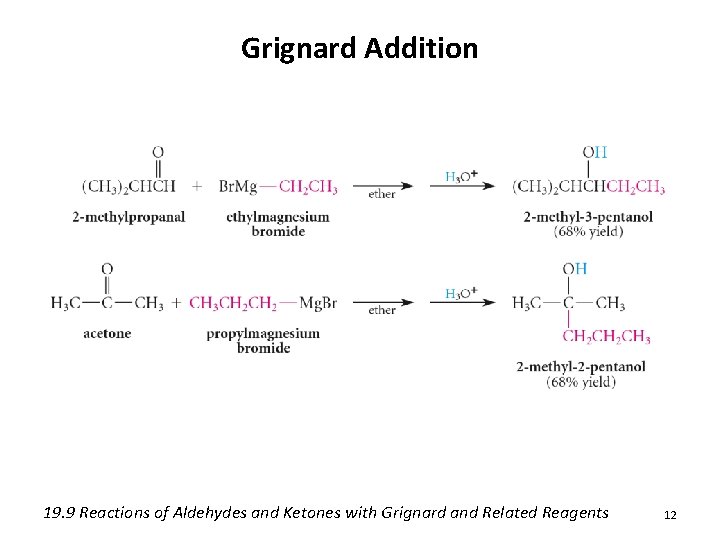
Grignard Addition 19. 9 Reactions of Aldehydes and Ketones with Grignard and Related Reagents 12

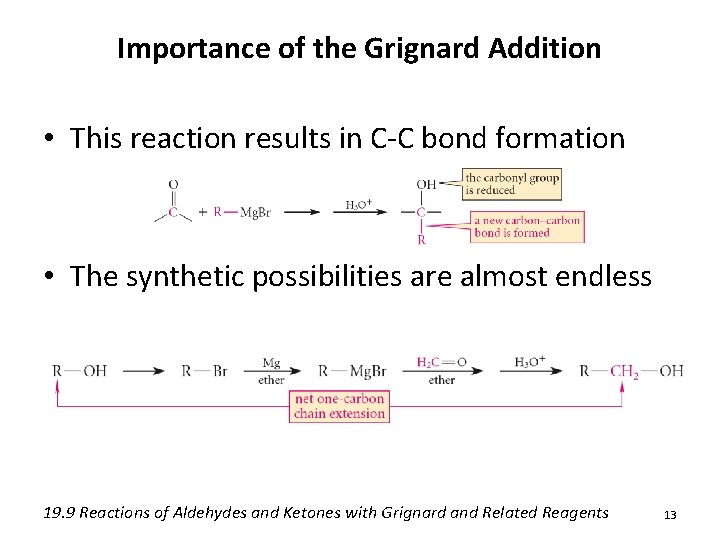
Importance of the Grignard Addition • This reaction results in C-C bond formation • The synthetic possibilities are almost endless 19. 9 Reactions of Aldehydes and Ketones with Grignard and Related Reagents 13

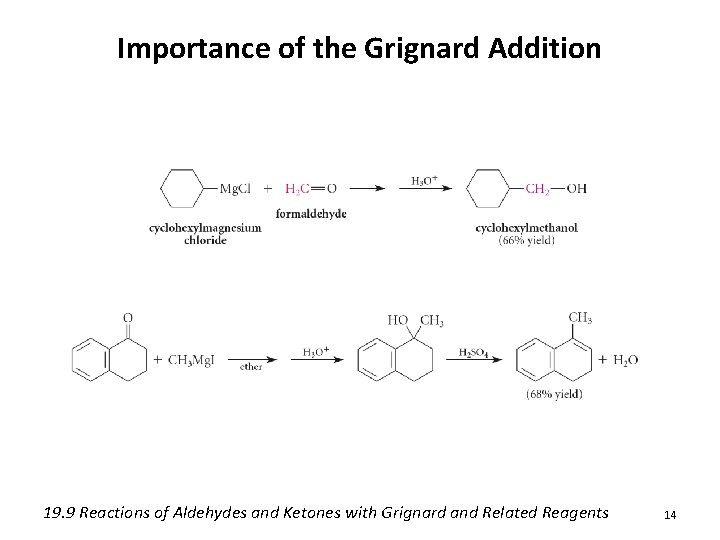
Importance of the Grignard Addition 19. 9 Reactions of Aldehydes and Ketones with Grignard and Related Reagents 14

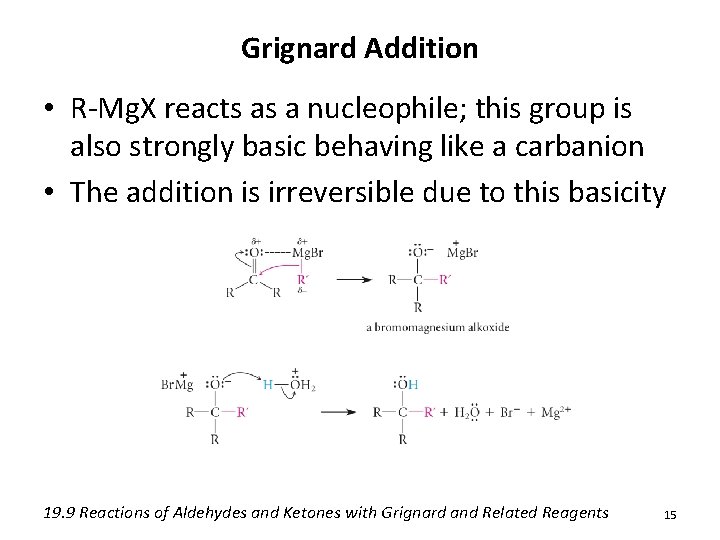
Grignard Addition • R-Mg. X reacts as a nucleophile; this group is also strongly basic behaving like a carbanion • The addition is irreversible due to this basicity 19. 9 Reactions of Aldehydes and Ketones with Grignard and Related Reagents 15

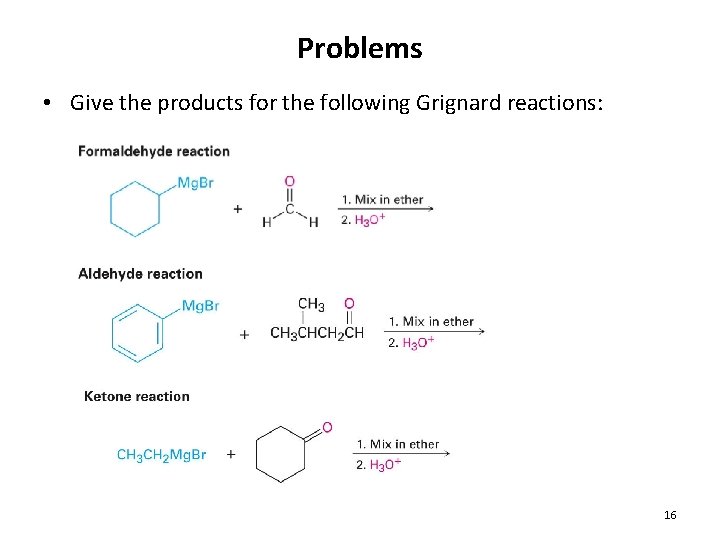
Problems • Give the products for the following Grignard reactions: 16

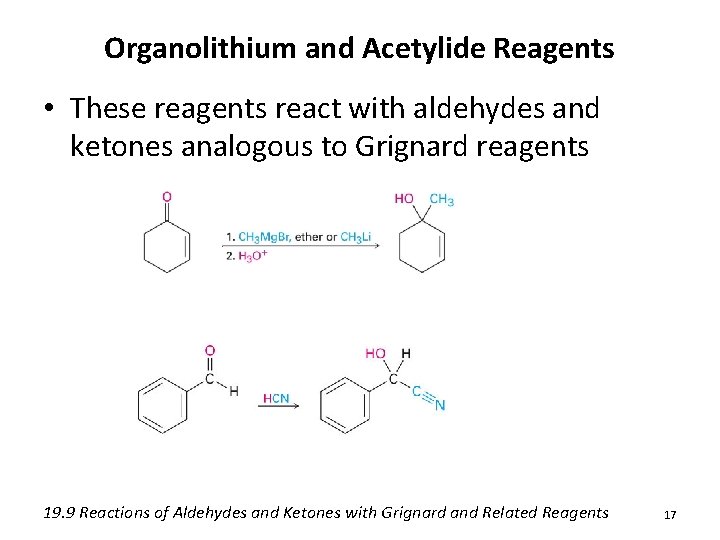
Organolithium and Acetylide Reagents • These reagents react with aldehydes and ketones analogous to Grignard reagents 19. 9 Reactions of Aldehydes and Ketones with Grignard and Related Reagents 17

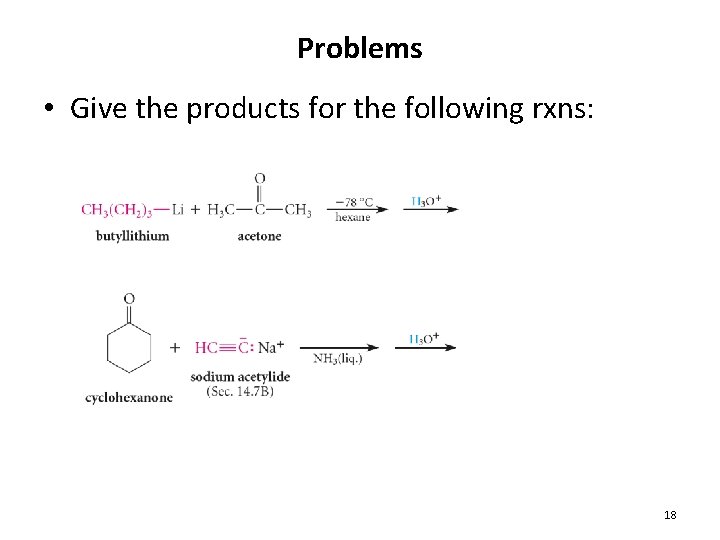
Problems • Give the products for the following rxns: 18

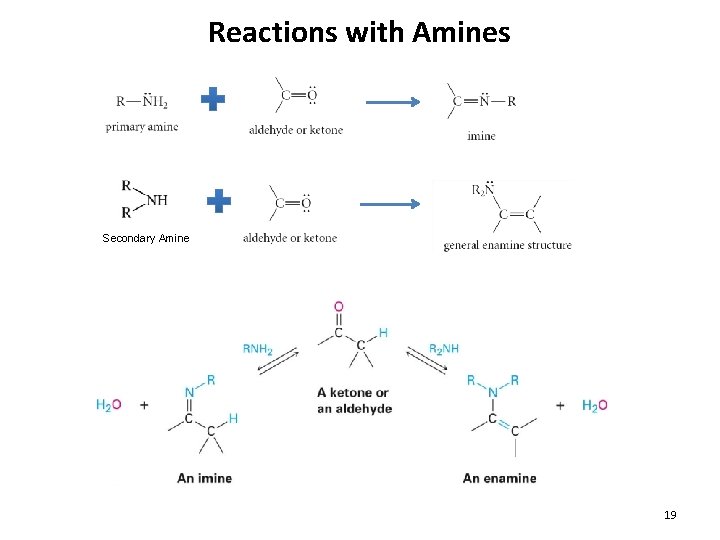
Reactions with Amines Secondary Amine 19

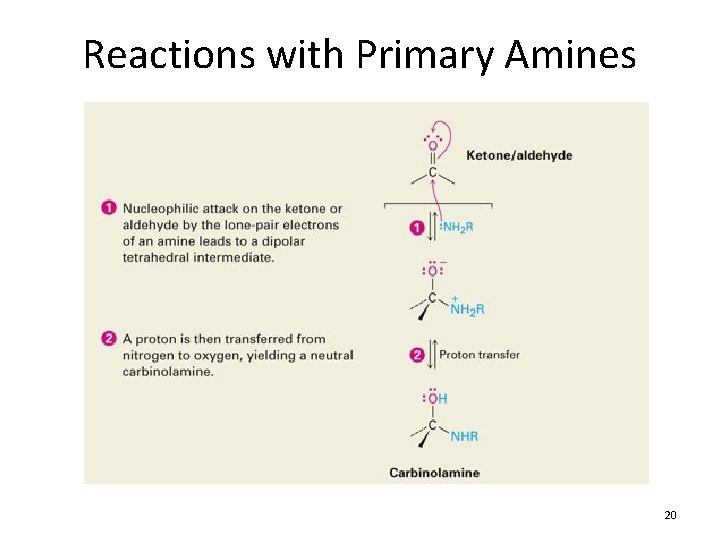
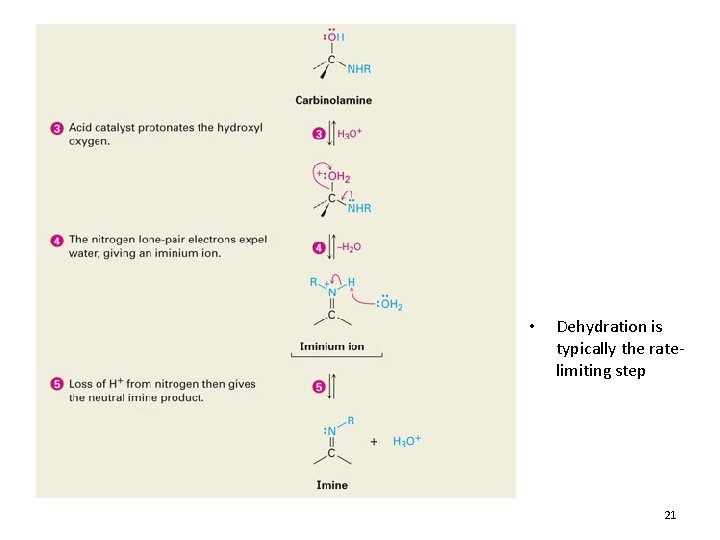
Reactions with Primary Amines 20

• Dehydration is typically the ratelimiting step 21

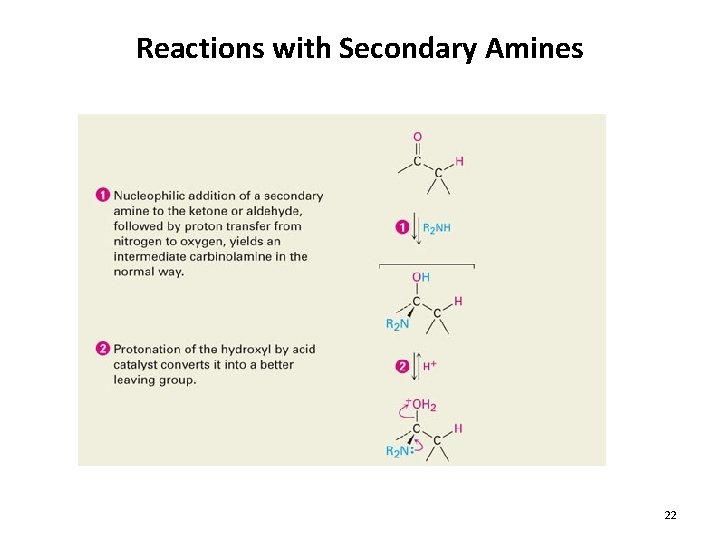
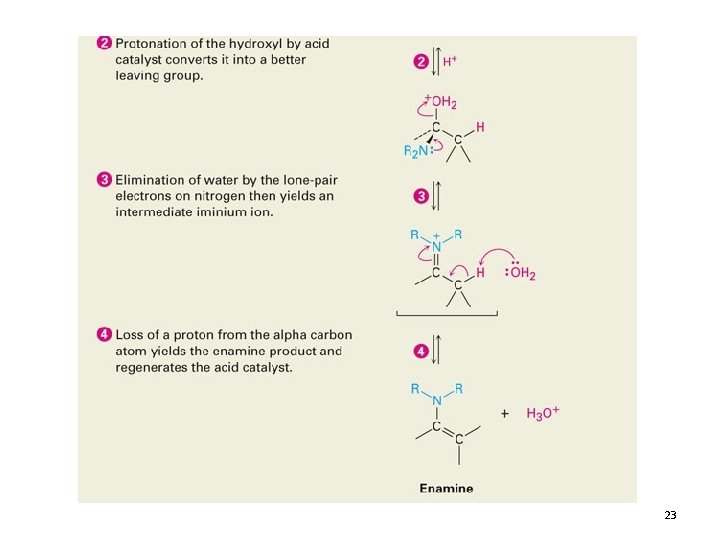
Reactions with Secondary Amines 22

23

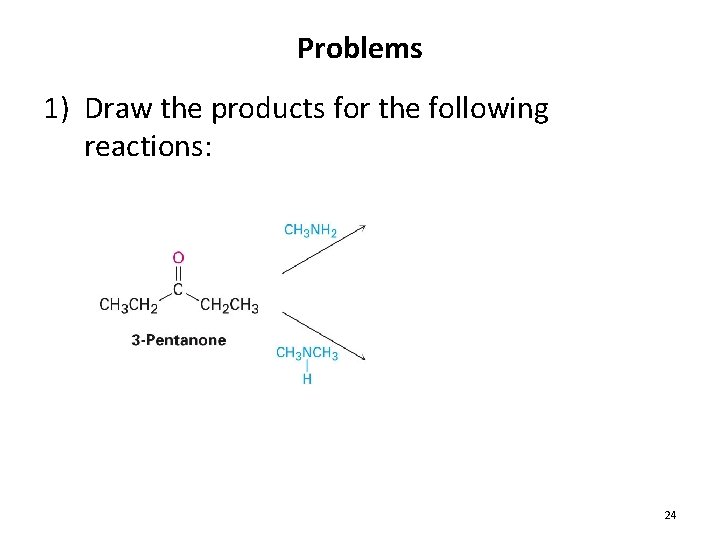
Problems 1) Draw the products for the following reactions: 24

2) Draw the mechanism for the formation of the imine in the previous question 25