Reduction Method of Spent Resin Generated from SG

- Slides: 25

Reduction Method of Spent Resin Generated from SG BD Ion Exchangers of PWR NPPS SEP. 12, 2007 Sung, Ki-Bang kbsung@khnp. co. kr 1

Contents 1. Introduction 2. Review of the early SGBD IX Replace Criteria 3. Experiment of IX Resin Capacity 4. Review of Experimental Results 5. Conclusions 2

KHNP’s R&D Institute (1) 3

KHNP’s R&D Institute (2) 4

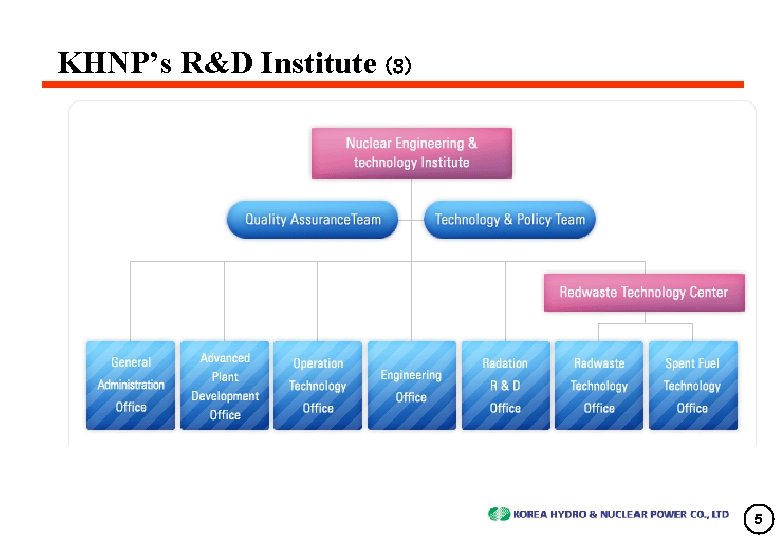
KHNP’s R&D Institute (3) 5

1. Introduction (1) ○ Background In Project of Kori #3, 4 NPP PSR, ü Main system of LLW Resin : SG BD Demineralizer § Cause : Secondary Side water p. H control agent : NH 3 ⇒ ETA-AVT The SGBD cation loads was increased about 2~3 times ü Spent Resin Radwaste : Large volume and no Industrial waste… Not easy to treat the ash, though spent resin is almost disposal object itself PSR Team treated as safety issue item ü PSR Team(NETEC) and Kori 2 Chemistry Section agreed to the problems and solved the sophisticated problems ü 6

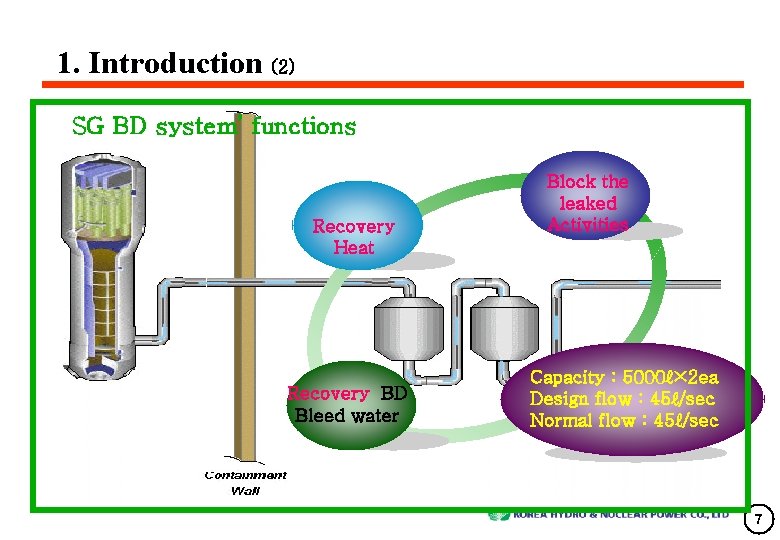
1. Introduction (2) SG BD system’ functions Recovery Heat Recovery BD Bleed water Block the leaked Activities Capacity : 5000ℓ× 2 ea Design flow : 45ℓ/sec Normal flow : 45ℓ/sec 7

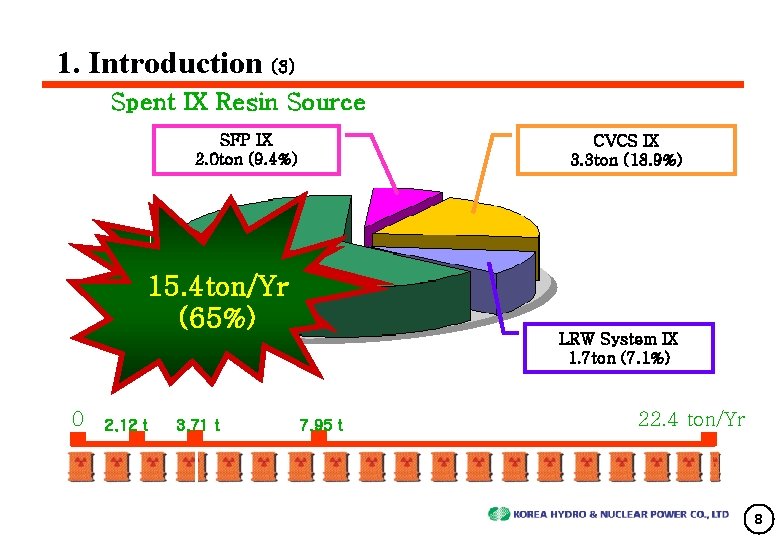
1. Introduction (3) Spent IX Resin Source SFP IX 2. 0 ton (9. 4%) CVCS IX 3. 3 ton (18. 9%) SG BD Demin 15. 4 ton/Yr (65%) 0 2. 12 t 3. 71 t LRW System IX 1. 7 ton (7. 1%) 7. 95 t 22. 4 ton/Yr 8

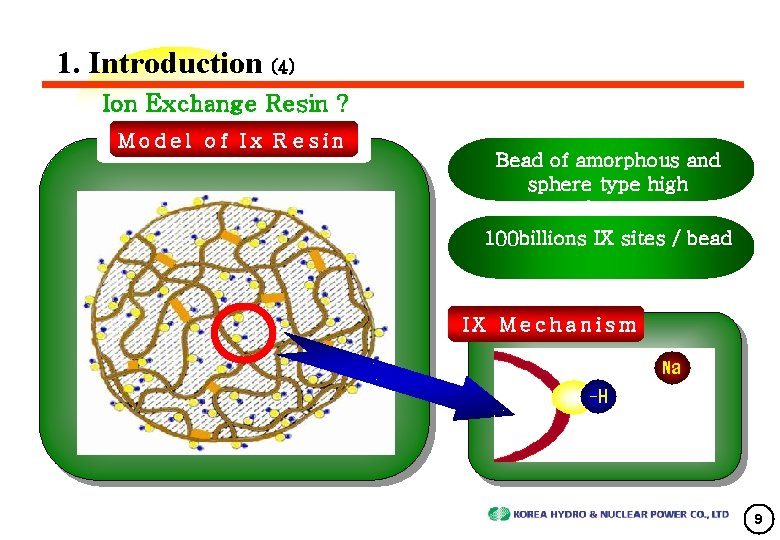
1. Introduction (4) Ion Exchange Resin ? Model of Ix R esin Bead of amorphous and sphere type high polymers 100 billions IX sites / bead IX Mecha n ism 9

2. Review of the early SGBD IX Replace Criteria (1) In Domestic Plants, • IX Resin Replacement Procedures of SGBD IX - No standard Criteria of SGBD IX - Na was a typical ion to determine the IX removal capacity in many plants. - However, Na was not a typical ion to determine IX removal capacity other plants ( See the next page table ) In USA NPPs, (from EPRI report) • IX Resin Replacing Procedures of SGBD IX - 22 of 73 PWR plant didn’t consider the Na ion as IX replacement Criteria 10

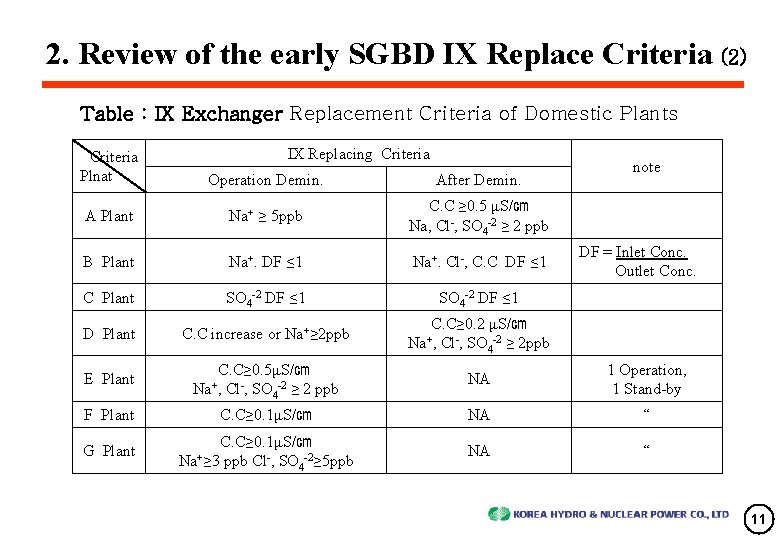
2. Review of the early SGBD IX Replace Criteria (2) Table : IX Exchanger Replacement Criteria of Domestic Plants IX Replacing Criteria Plnat Operation Demin. After Demin. A Plant Na+ ≥ 5 ppb C. C ≥ 0. 5 μS/㎝ Na, Cl-, SO 4 -2 ≥ 2 ppb B Plant Na+. DF ≤ 1 Na+. Cl-, C. C DF ≤ 1 DF = Inlet Conc. Outlet Conc. C Plant SO 4 -2 DF ≤ 1 D Plant C. C increase or Na+≥ 2 ppb E Plant C. C≥ 0. 5μS/㎝ Na+, Cl-, SO 4 -2 ≥ 2 ppb NA 1 Operation, 1 Stand-by F Plant C. C≥ 0. 1μS/㎝ NA “ G Plant C. C≥ 0. 1μS/㎝ Na+≥ 3 ppb Cl-, SO 4 -2≥ 5 ppb NA “ C. C≥ 0. 2 μS/㎝ Na+, Cl-, SO 4 -2 ≥ 2 ppb note 11

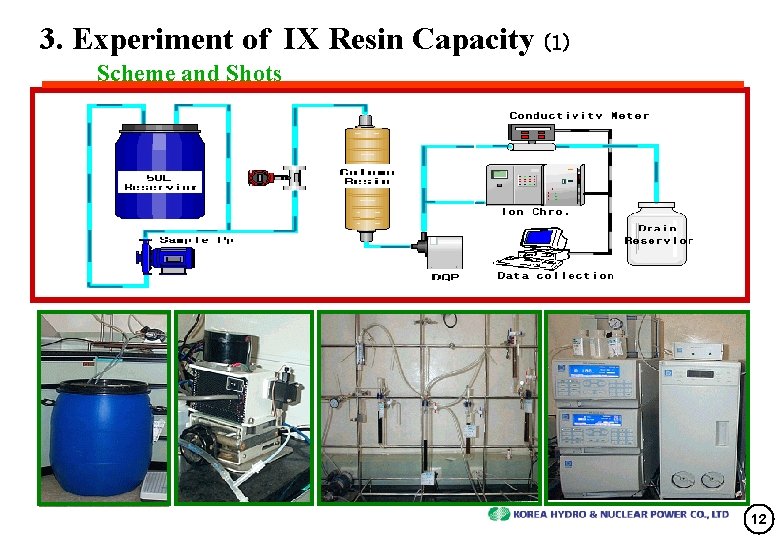
3. Experiment of IX Resin Capacity (1) Scheme and Shots 12

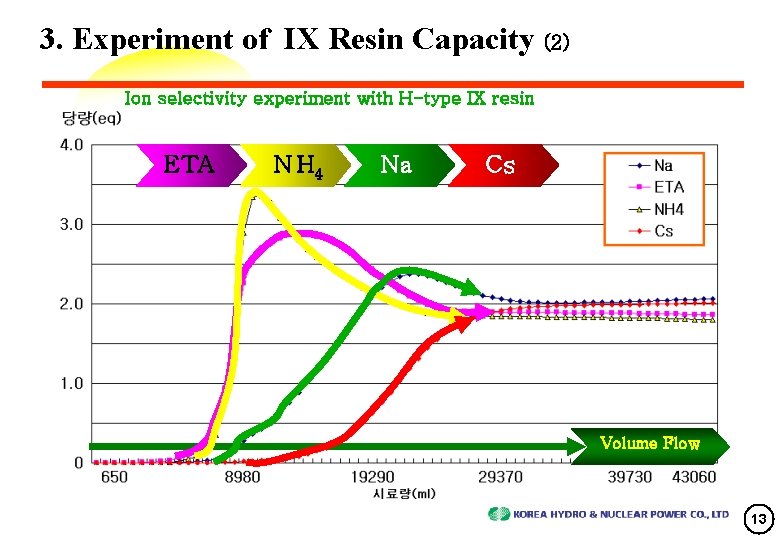
3. Experiment of IX Resin Capacity (2) Ion selectivity experiment with H-type IX resin ETA N H 4 Na Cs Volume Flow 13

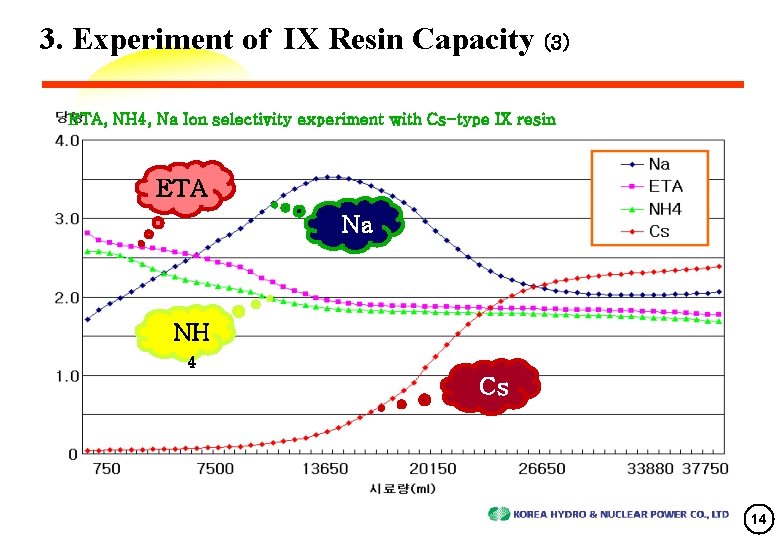
3. Experiment of IX Resin Capacity (3) ETA, NH 4, Na Ion selectivity experiment with Cs-type IX resin ETA Na NH 4 Cs 14

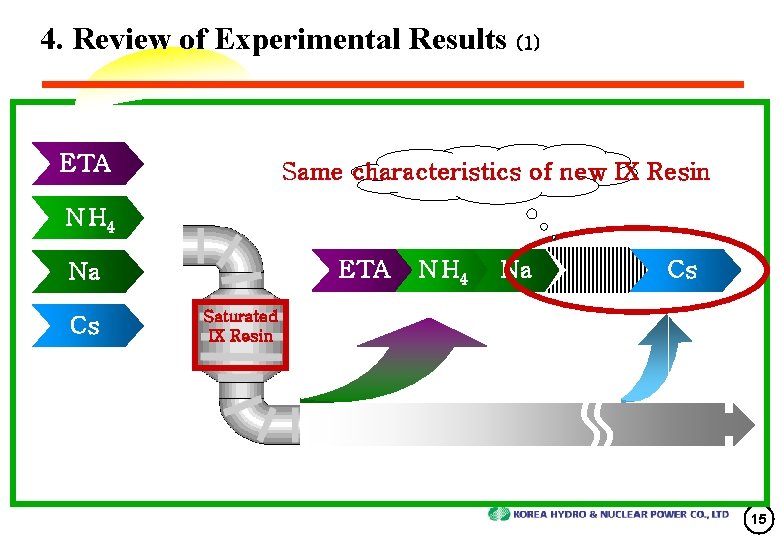
4. Review of Experimental Results (1) ETA Same characteristics of new IX Resin N H 4 ETA Na Cs N H 4 Na Cs Saturated IX Resin 15

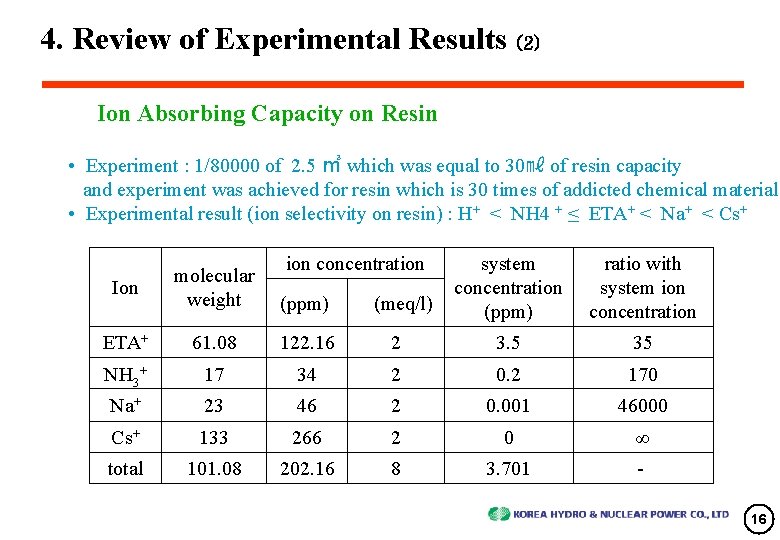
4. Review of Experimental Results (2) Ion Absorbing Capacity on Resin • Experiment : 1/80000 of 2. 5 ㎥ which was equal to 30㎖ of resin capacity and experiment was achieved for resin which is 30 times of addicted chemical material • Experimental result (ion selectivity on resin) : H+ < NH 4 + ≤ ETA+ < Na+ < Cs+ ion concentration system concentration (ppm) ratio with system ion concentration Ion molecular weight ETA+ 61. 08 122. 16 2 3. 5 35 NH 3+ 17 34 2 0. 2 170 Na+ 23 46 2 0. 001 46000 Cs+ 133 266 2 0 ∞ total 101. 08 202. 16 8 3. 701 - (ppm) (meq/l) 16

4. Review of Experimental Results (3) Ion Breakthrough Curve Characteristics • ETA 0. 5 [Output conc. /Input conc. ] Breakthrough time(T) -> (ETA+NH 3) : Na : Cs = 2 T : 3 T : 4 T This phenomenon came from ETA/NH 3, Na and Cs ‘s different selectiveness. • NH 3 was eluted after ETA and exchange reaction was faster than ETA. ※ NH 3 was produced from ETA or N 2 H 4 which removes Oxygen. • Na’s sensitivity was stronger than NH 3 or ETA. So, Na was eluted after NH 3 or ETA. High Peak position of Na Conc. was overrode on Cs’s conc. 1. 0 meq/ℓ(half input Conc. ) ⇒ Cs extrude Na • Cs has S-shape breakthrough characteristics like single ion and it was absorbed on IX resin. Cs was not affected by other ions, and Its behavior look like single ion solution. 17

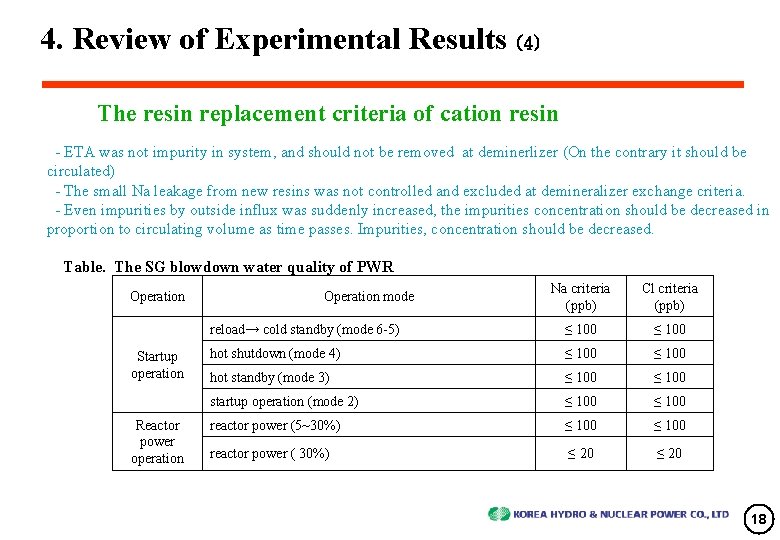
4. Review of Experimental Results (4) The resin replacement criteria of cation resin - ETA was not impurity in system, and should not be removed at deminerlizer (On the contrary it should be circulated) - The small Na leakage from new resins was not controlled and excluded at demineralizer exchange criteria. - Even impurities by outside influx was suddenly increased, the impurities concentration should be decreased in proportion to circulating volume as time passes. Impurities, concentration should be decreased. Table. The SG blowdown water quality of PWR Operation Startup operation Reactor power operation Na criteria (ppb) Cl criteria (ppb) reload→ cold standby (mode 6 -5) ≤ 100 hot shutdown (mode 4) ≤ 100 hot standby (mode 3) ≤ 100 startup operation (mode 2) ≤ 100 reactor power (5~30%) ≤ 100 reactor power ( 30%) ≤ 20 Operation mode 18

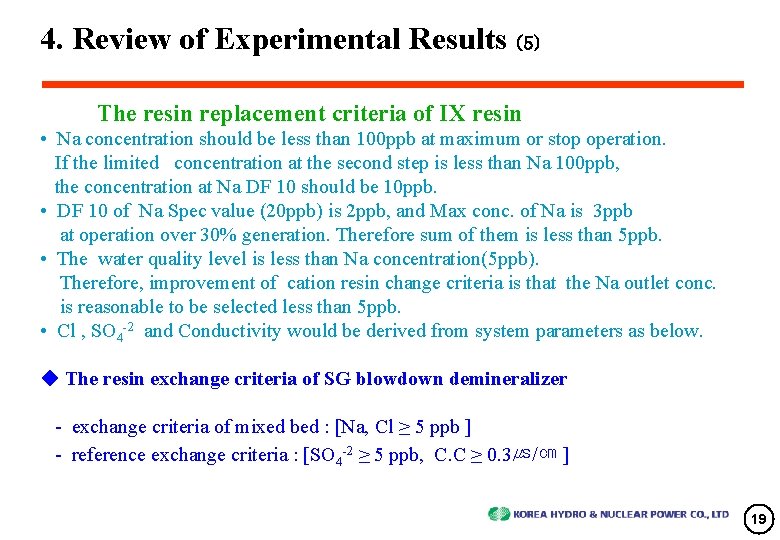
4. Review of Experimental Results (5) The resin replacement criteria of IX resin • Na concentration should be less than 100 ppb at maximum or stop operation. If the limited concentration at the second step is less than Na 100 ppb, the concentration at Na DF 10 should be 10 ppb. • DF 10 of Na Spec value (20 ppb) is 2 ppb, and Max conc. of Na is 3 ppb at operation over 30% generation. Therefore sum of them is less than 5 ppb. • The water quality level is less than Na concentration(5 ppb). Therefore, improvement of cation resin change criteria is that the Na outlet conc. is reasonable to be selected less than 5 ppb. • Cl , SO 4 -2 and Conductivity would be derived from system parameters as below. ◆ The resin exchange criteria of SG blowdown demineralizer - exchange criteria of mixed bed : [Na, Cl ≥ 5 ppb ] - reference exchange criteria : [SO 4 -2 ≥ 5 ppb, C. C ≥ 0. 3㎲/㎝ ] 19

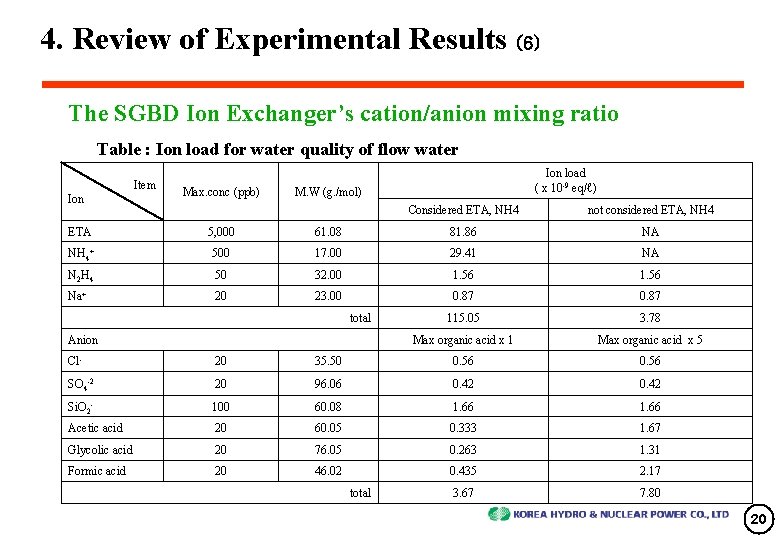
4. Review of Experimental Results (6) The SGBD Ion Exchanger’s cation/anion mixing ratio Table : Ion load for water quality of flow water Item Ion Max. conc (ppb) Ion load ( x 10 -9 eq/ℓ) M. W (g. /mol) Considered ETA, NH 4 not considered ETA, NH 4 ETA 5, 000 61. 08 81. 86 NA NH 4+ 500 17. 00 29. 41 NA N 2 H 4 50 32. 00 1. 56 Na+ 20 23. 00 0. 87 115. 05 3. 78 Max organic acid x 1 Max organic acid x 5 total Anion Cl- 20 35. 50 0. 56 SO 4 -2 20 96. 06 0. 42 Si. O 2 - 100 60. 08 1. 66 Acetic acid 20 60. 05 0. 333 1. 67 Glycolic acid 20 76. 05 0. 263 1. 31 Formic acid 20 46. 02 0. 435 2. 17 3. 67 7. 80 total 20

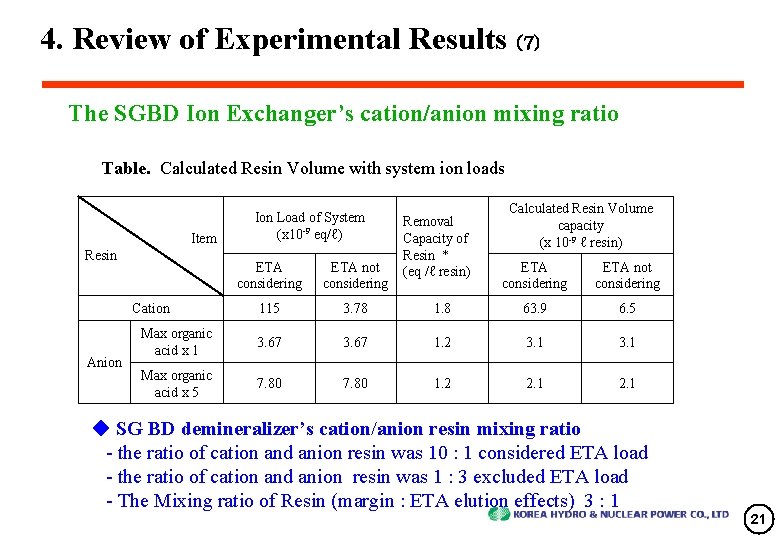
4. Review of Experimental Results (7) The SGBD Ion Exchanger’s cation/anion mixing ratio Table. Calculated Resin Volume with system ion loads Item Resin ETA considering ETA not considering 115 3. 78 Max organic acid x 1 3. 67 Max organic acid x 5 7. 80 Cation Anion Ion Load of System (x 10 -9 eq/ℓ) Removal Capacity of Resin * (eq /ℓ resin) Calculated Resin Volume capacity (x 10 -9 ℓ resin) ETA considering ETA not considering 1. 8 63. 9 6. 5 3. 67 1. 2 3. 1 7. 80 1. 2 2. 1 ◆ SG BD demineralizer’s cation/anion resin mixing ratio - the ratio of cation and anion resin was 10 : 1 considered ETA load - the ratio of cation and anion resin was 1 : 3 excluded ETA load - The Mixing ratio of Resin (margin : ETA elution effects) 3 : 1 21

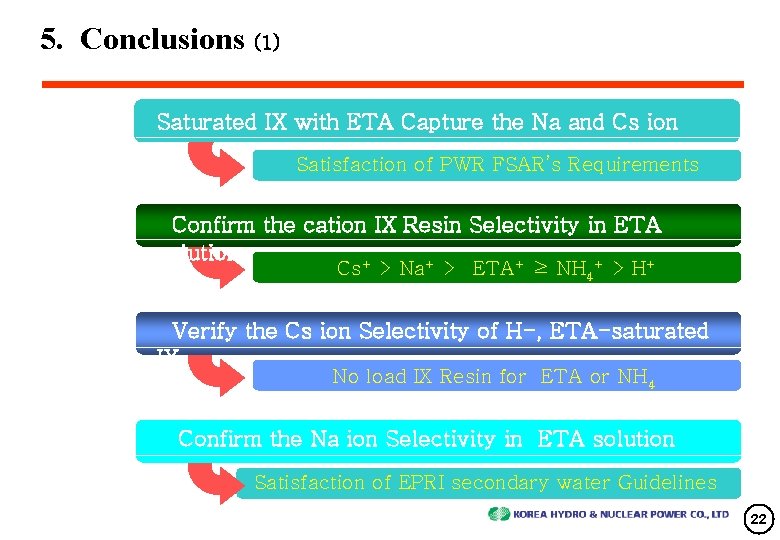
5. Conclusions (1) Saturated IX with ETA Capture the Na and Cs ion Satisfaction of PWR FSAR’s Requirements Confirm the cation IX Resin Selectivity in ETA solution + + + Cs > Na > ETA ≥ NH 4 > H Verify the Cs ion Selectivity of H-, ETA-saturated IX No load IX Resin for ETA or NH 4 Confirm the Na ion Selectivity in ETA solution Satisfaction of EPRI secondary water Guidelines 22

5. Conclusions (2) 2 1 Establish Spent Resin change Criteria Standardization Spent Resin Criteria and Mixing IX Resin Technical Effects 3 Obtain ion Selectivity for Htype ion 4 Examined Na’s behavior in system 23

5. Conclusions (3) Reduce Spent IX resin(370 t/yr) Save the Cost(3. 3 billion. Won/total Min. Low Level Radioactive Wastes Reduce works on Radiation area Min. Radiation Exposure Other Effects Water quality treatment tech. Max. removal Capacity of Resin Co-works Solving Problem example with another team 24

Thank you for your attentions ! 25