Redox Solutions of potassium iodide potassium iodate and




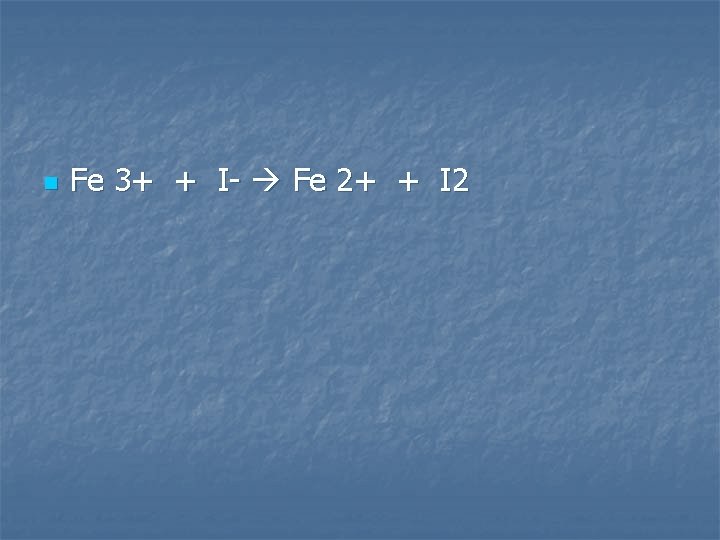

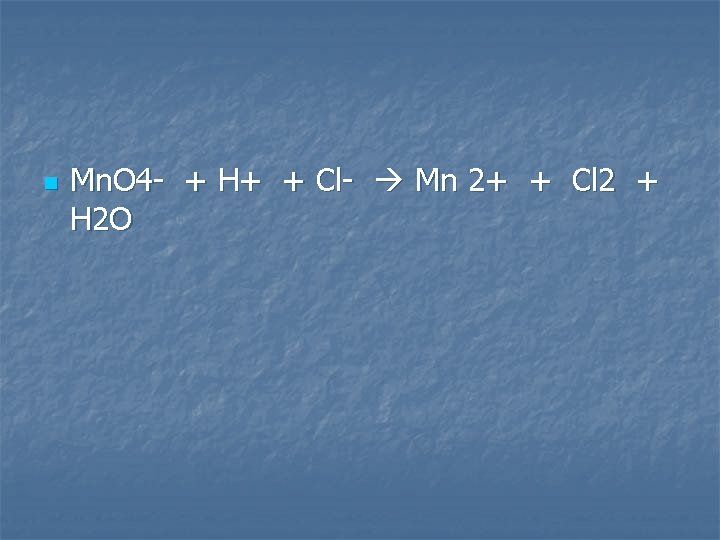

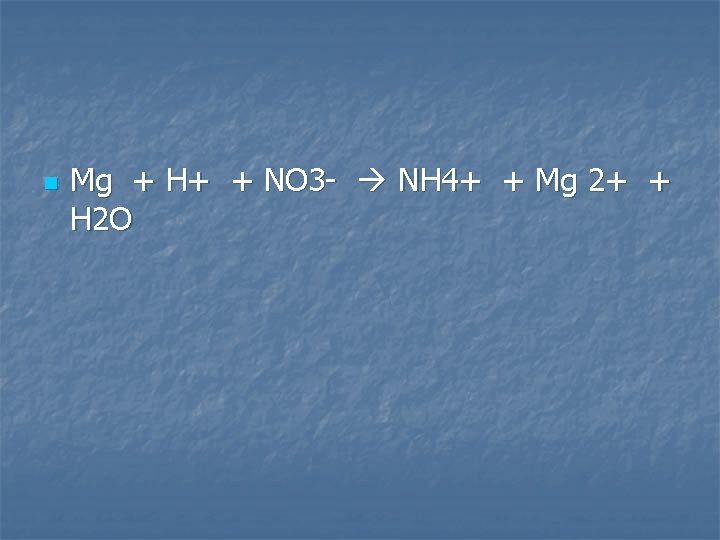

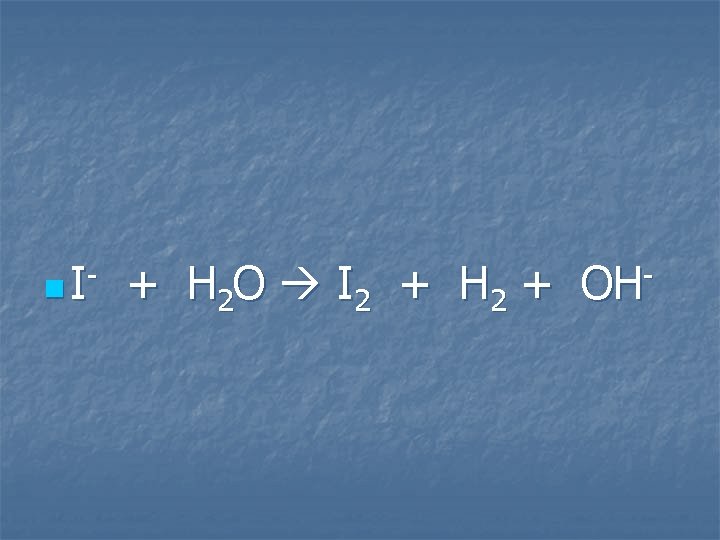

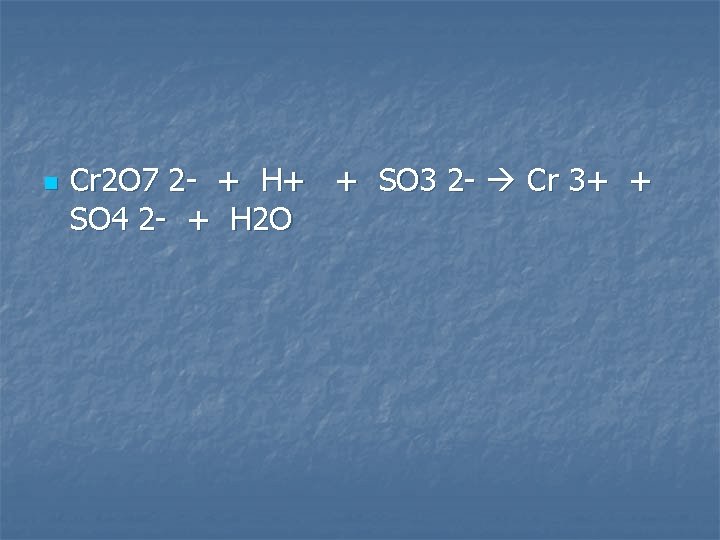

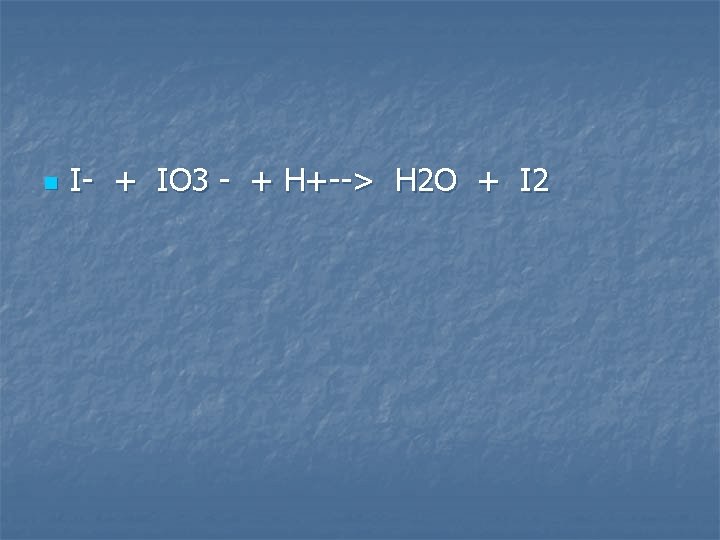

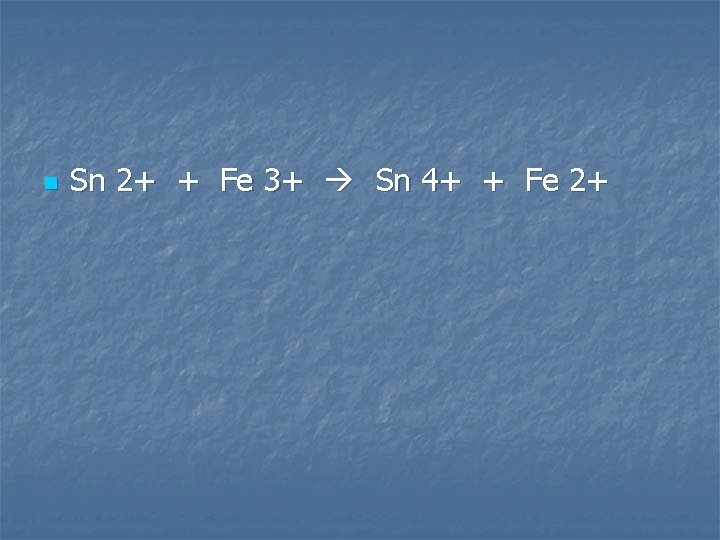

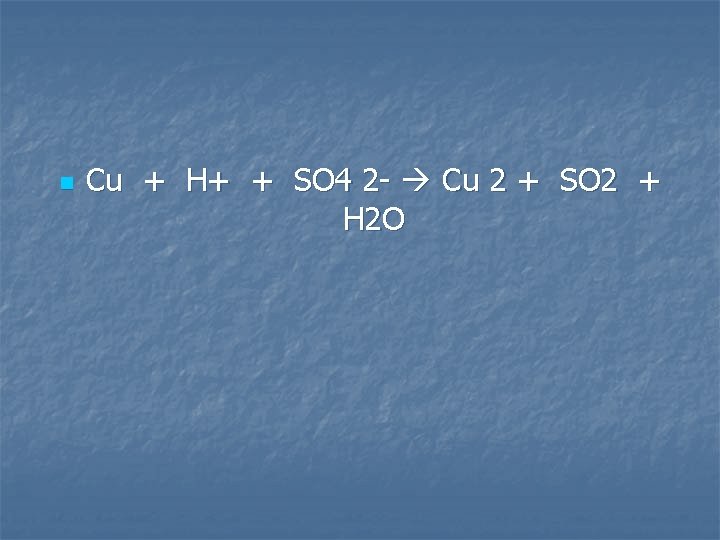

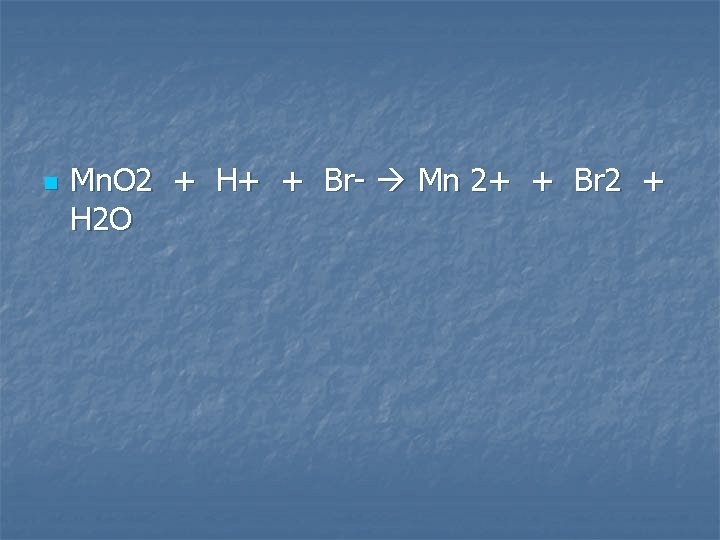

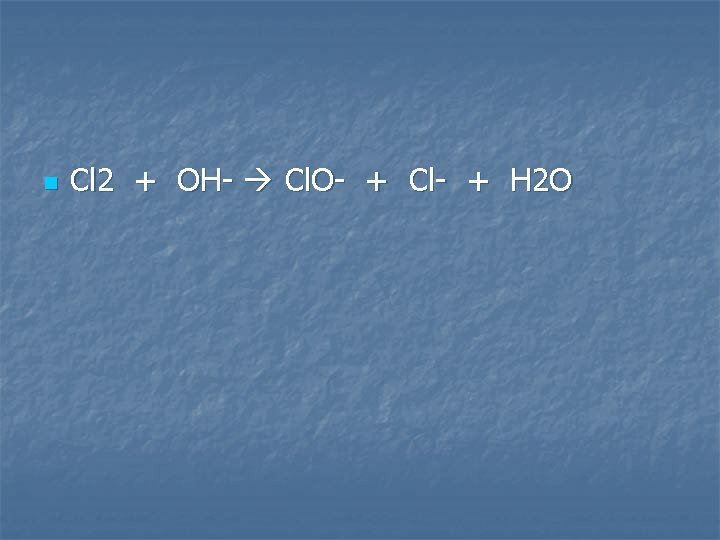

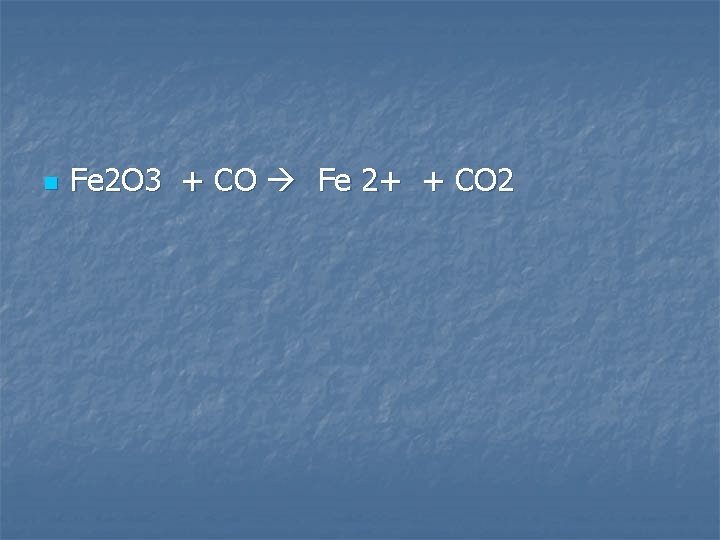

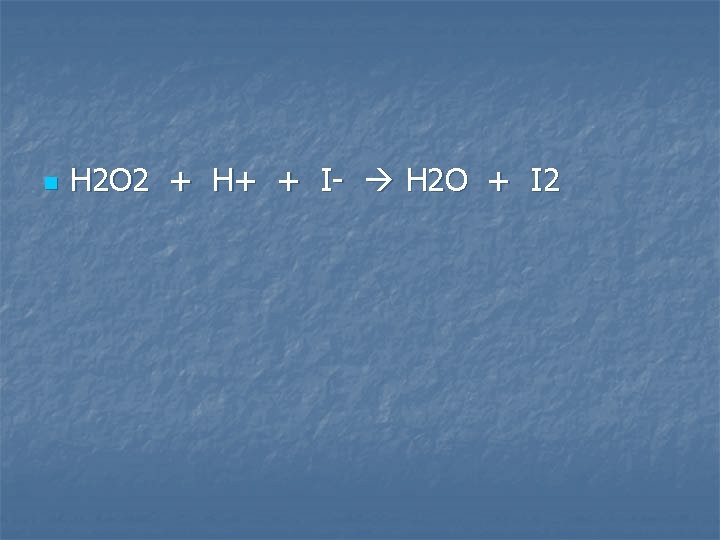

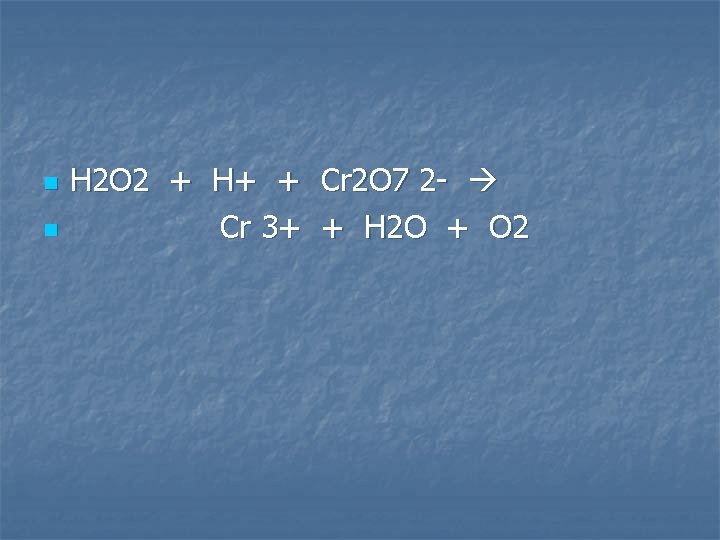

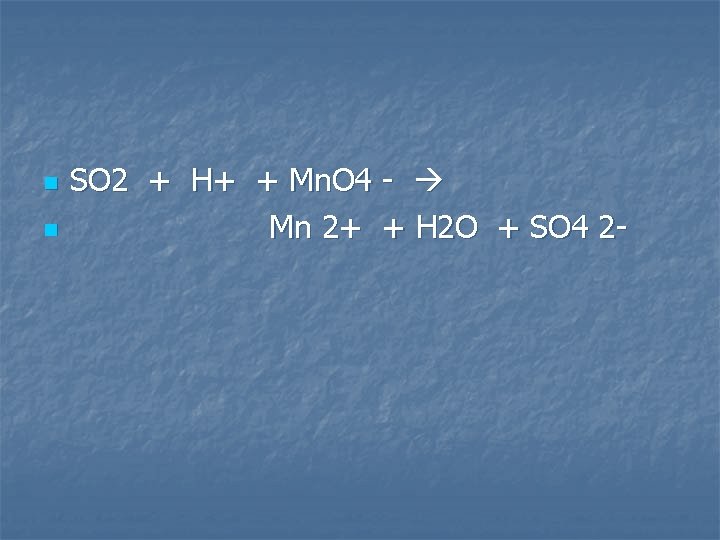

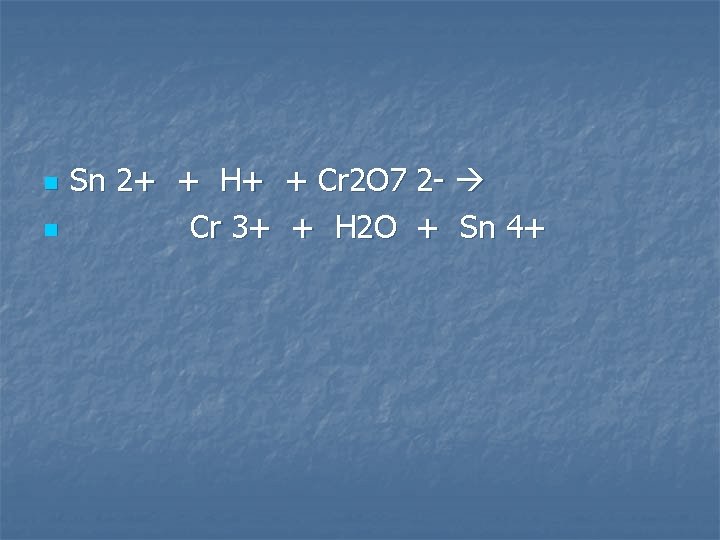

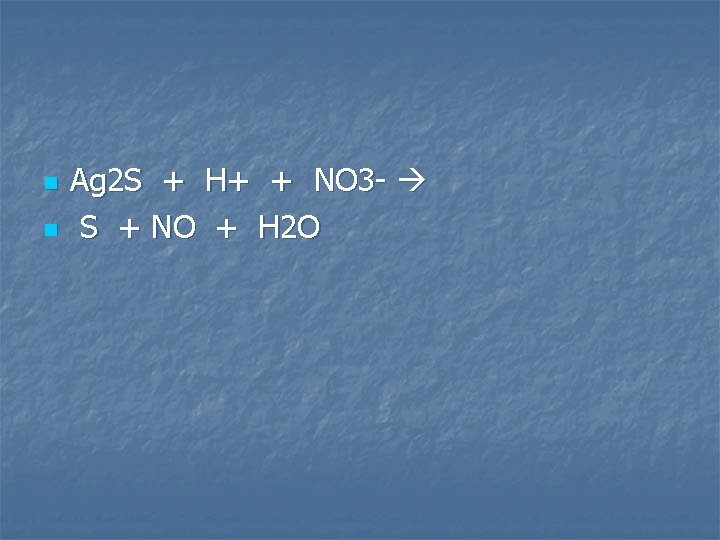



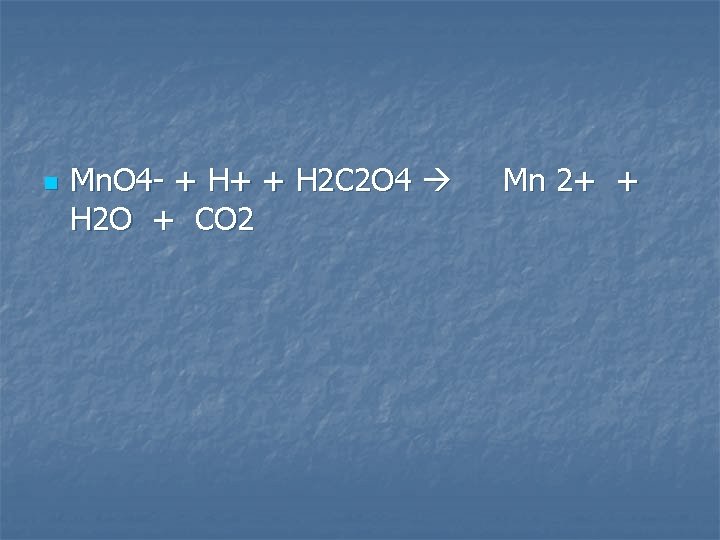

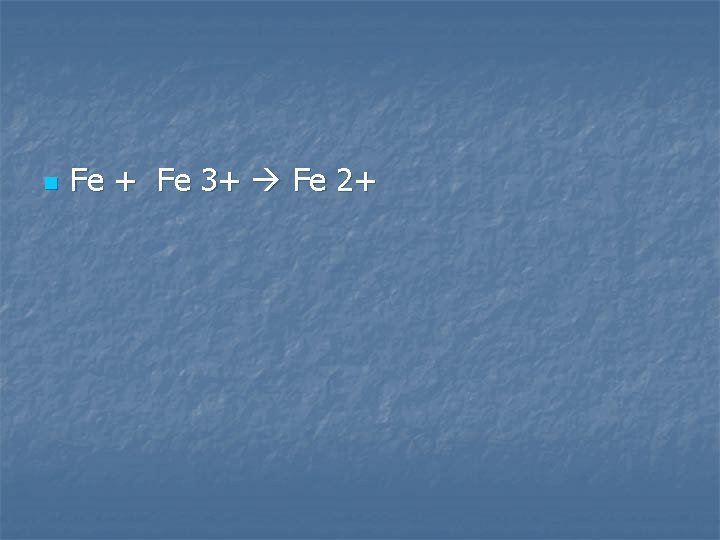



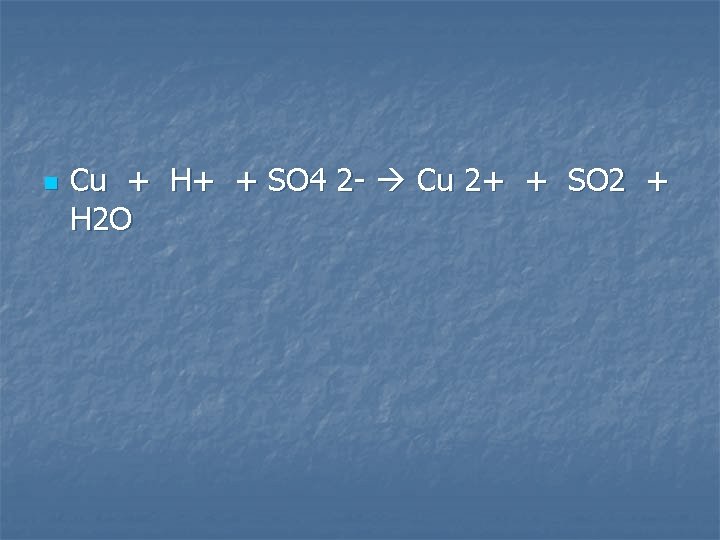


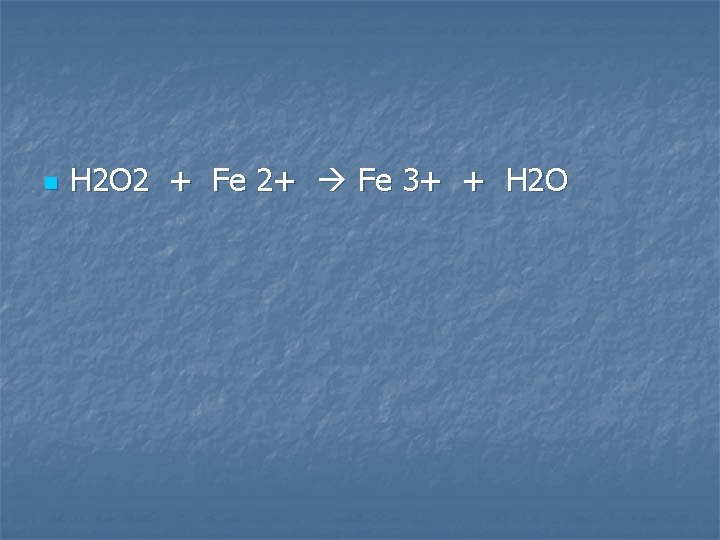

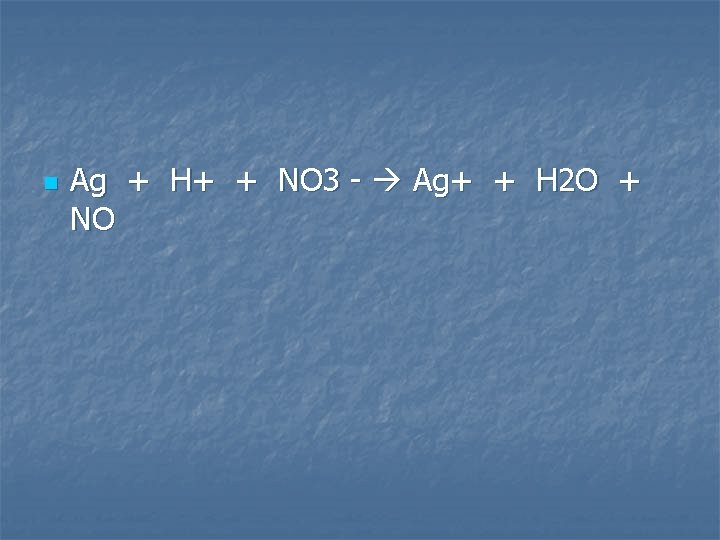

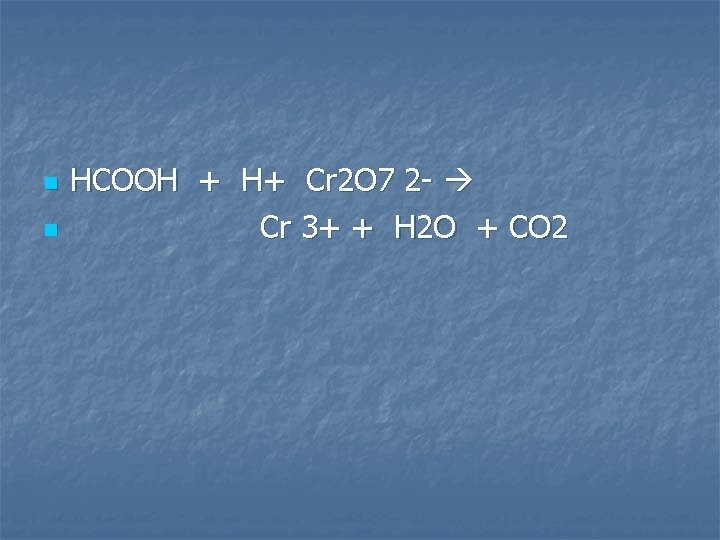

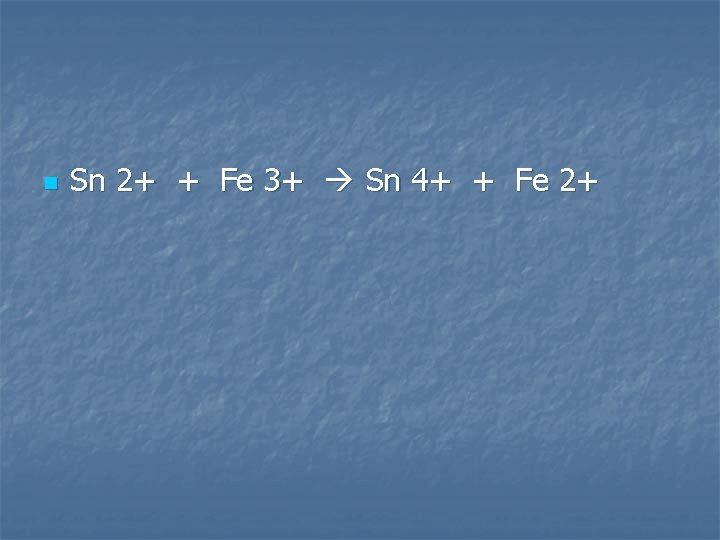
- Slides: 55

Redox

Solutions of potassium iodide, potassium iodate, and dilute sulfuric acid are mixed.

I-(aq) + IO 3 -(aq) + H+(aq) ® H 2 O + I 2(aq) [or I 3 -(aq)]

Iron (III) ions are reduced by iodide ions
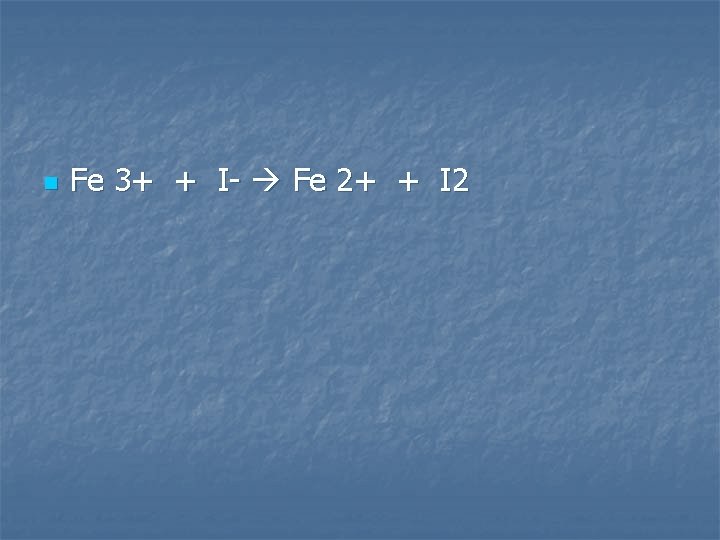
n Fe 3+ + I- Fe 2+ + I 2

Potassium permanganate solution is added to concentrated hydrochloric acid
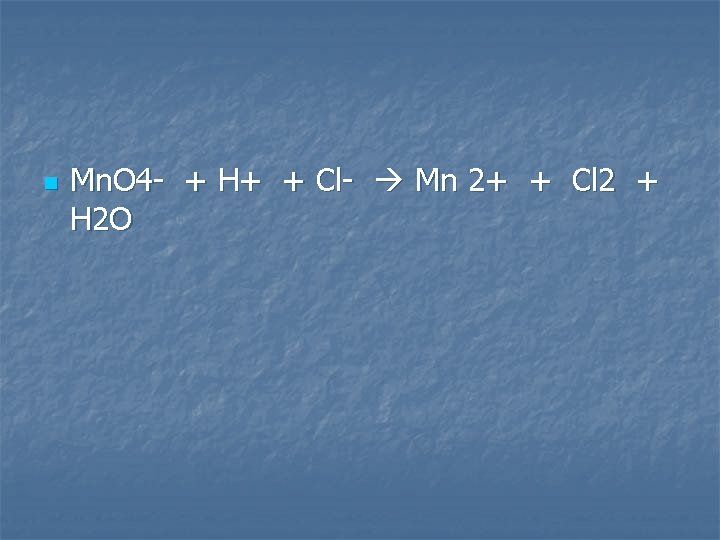
n Mn. O 4 - + H+ + Cl- Mn 2+ + Cl 2 + H 2 O

Magnesium metal is added to dilute nitric acid, giving as one of the products a compound in which the oxidation number for nitrogen is -3
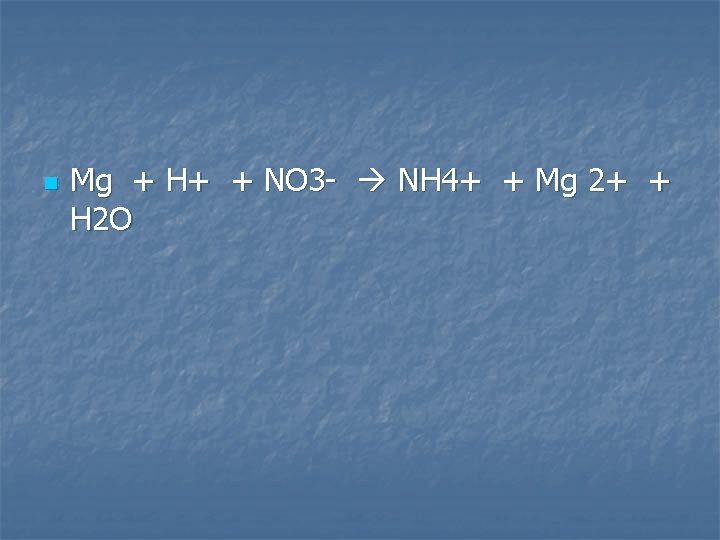
n Mg + H+ + NO 3 - NH 4+ + Mg 2+ + H 2 O

A solution of potassium iodide is electrolyzed
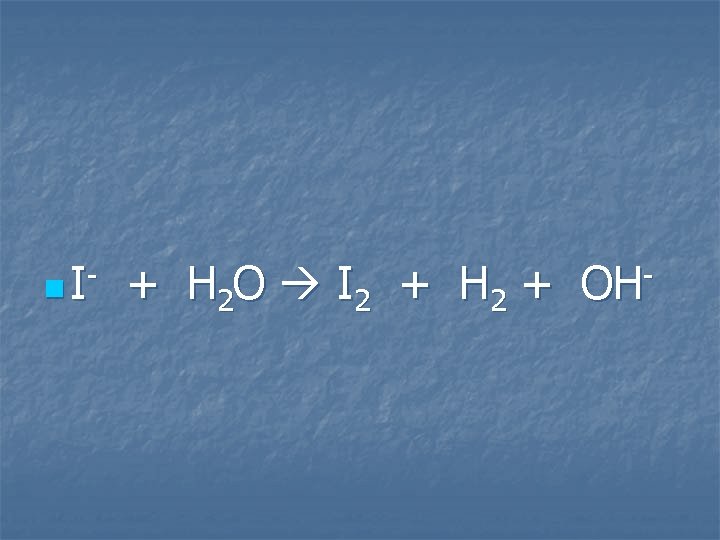
n I- + H 2 O I 2 + H 2 + OH-

Potassium dichromate solution is added to an acidified solution of sodium sulfite
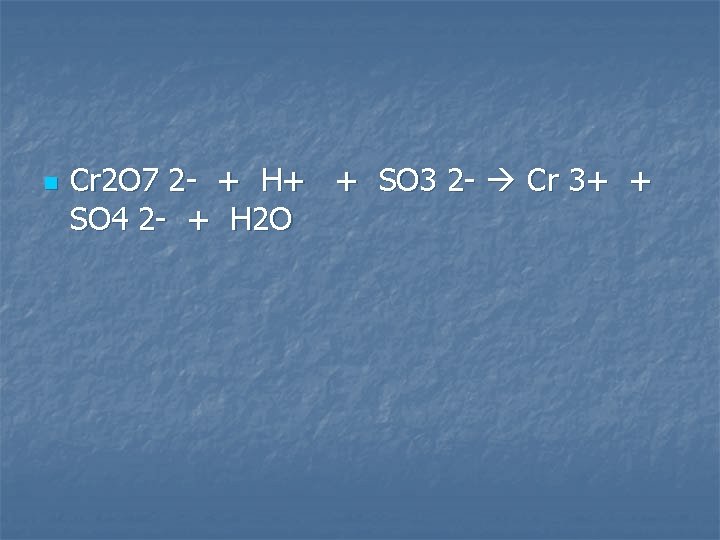
n Cr 2 O 7 2 - + H+ + SO 3 2 - Cr 3+ + SO 4 2 - + H 2 O

Solutions of potassium Iodide, potassium iodate, and dilute sulfuric acid are mixed
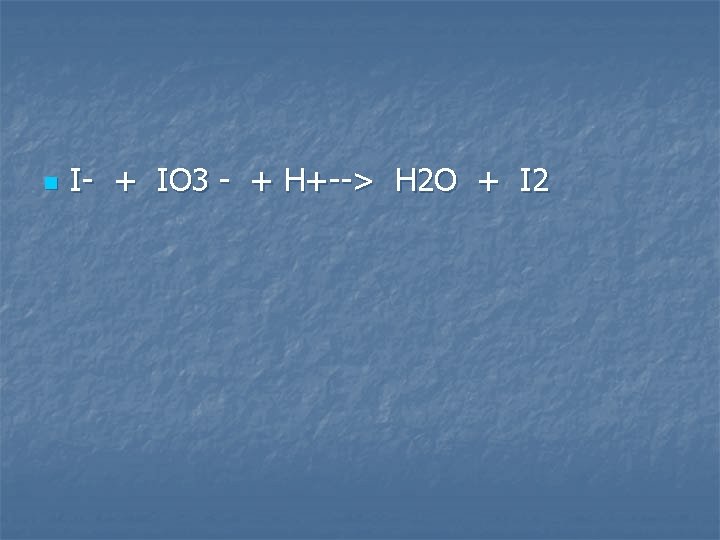
n I- + IO 3 - + H+--> H 2 O + I 2

Solutions of tin (II) sulfate is added to a solution of Iron (III) sulfate
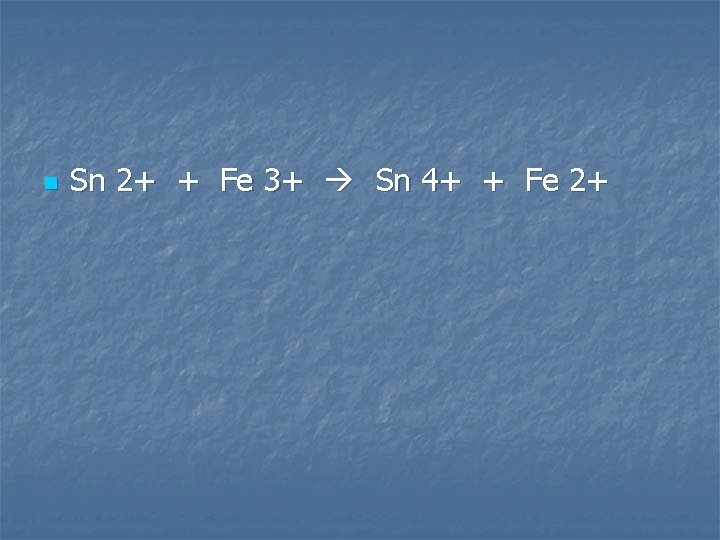
n Sn 2+ + Fe 3+ Sn 4+ + Fe 2+

Metallic copper is heated with concentrated sulfuric acid
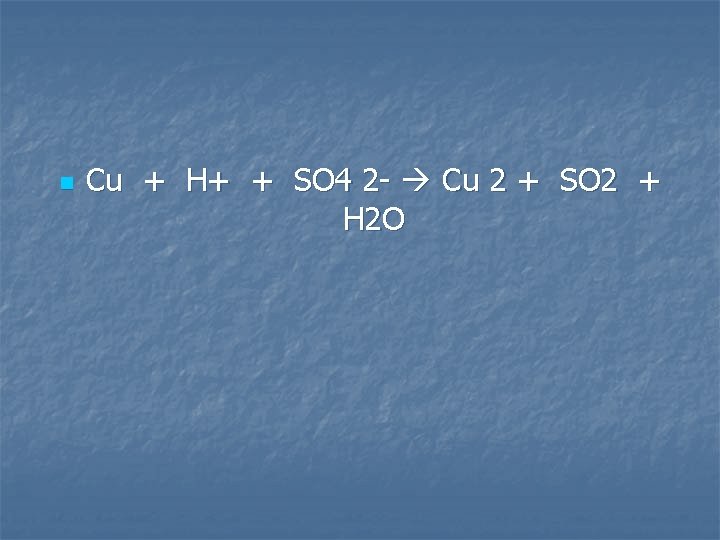
n Cu + H+ + SO 4 2 - Cu 2 + SO 2 + H 2 O

Manganese (IV) oxide is added to warm, concentrated hydrobromic acid
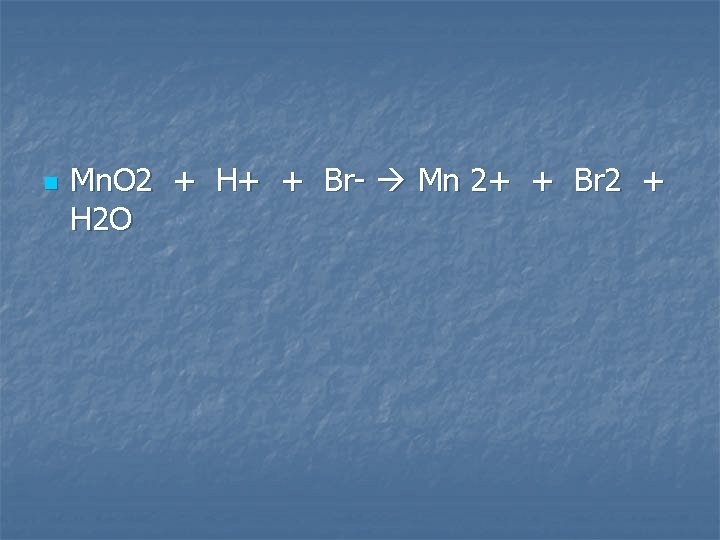
n Mn. O 2 + H+ + Br- Mn 2+ + Br 2 + H 2 O

Chlorine gas is bubbled into dilute sodium hydroxide
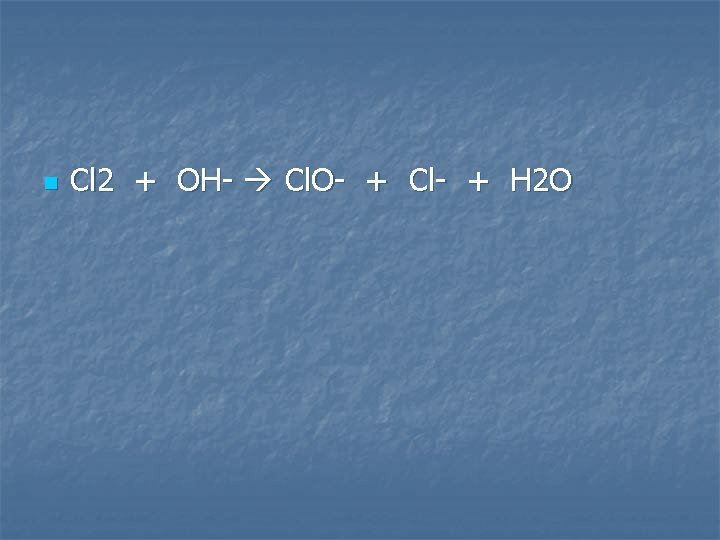
n Cl 2 + OH- Cl. O- + Cl- + H 2 O

Solid iron (III) oxide is heated in excess carbon monoxide
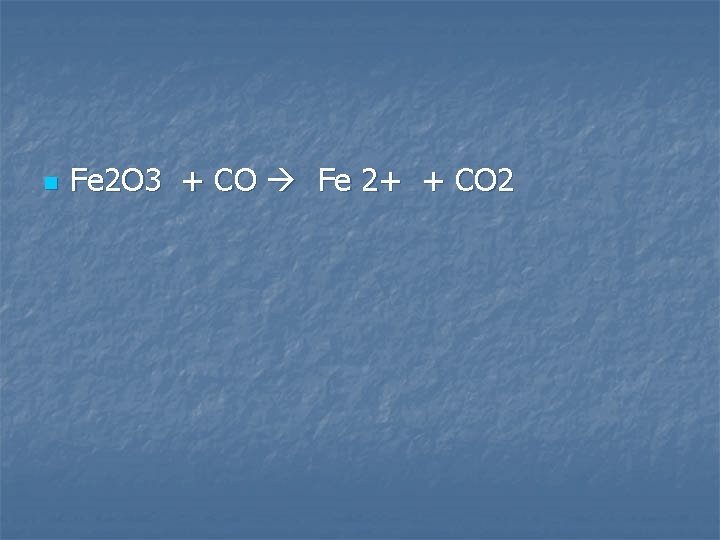
n Fe 2 O 3 + CO Fe 2+ + CO 2

Hydrogen peroxide solution is added to acidified potassium iodide solution
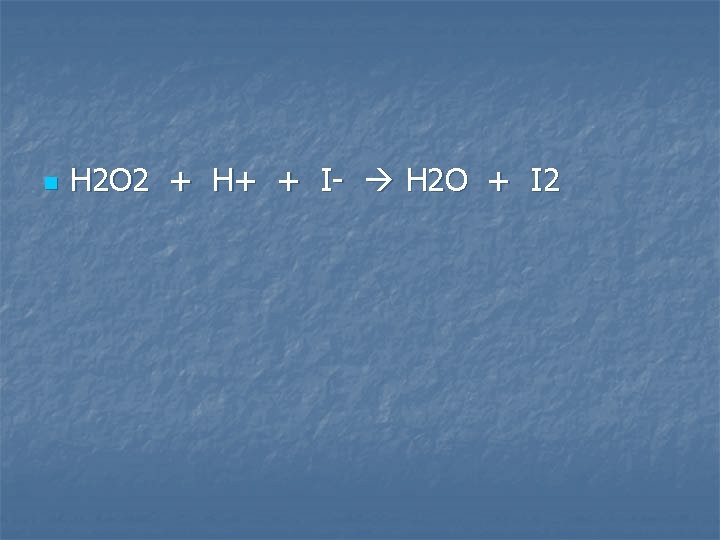
n H 2 O 2 + H+ + I- H 2 O + I 2

Hydrogen peroxide is added to an acidified solution of potassium dichromate
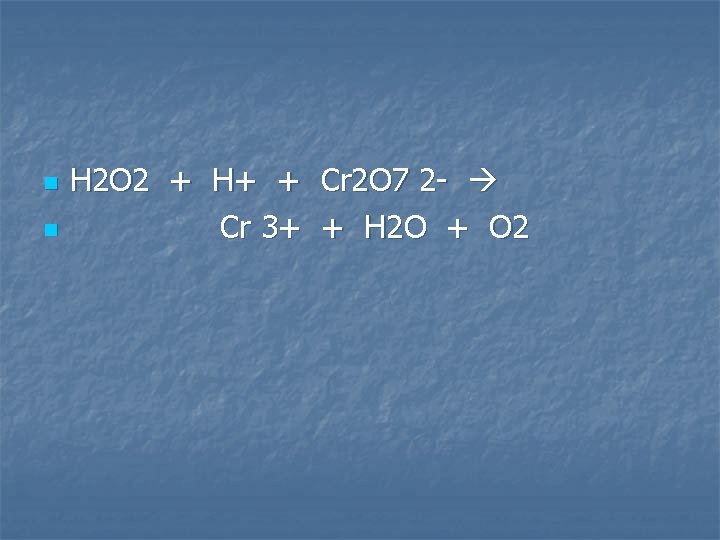
n n H 2 O 2 + H+ + Cr 2 O 7 2 - Cr 3+ + H 2 O + O 2

Sulfur dioxide gas is bubbled through an acidified solution of potassium permanganate
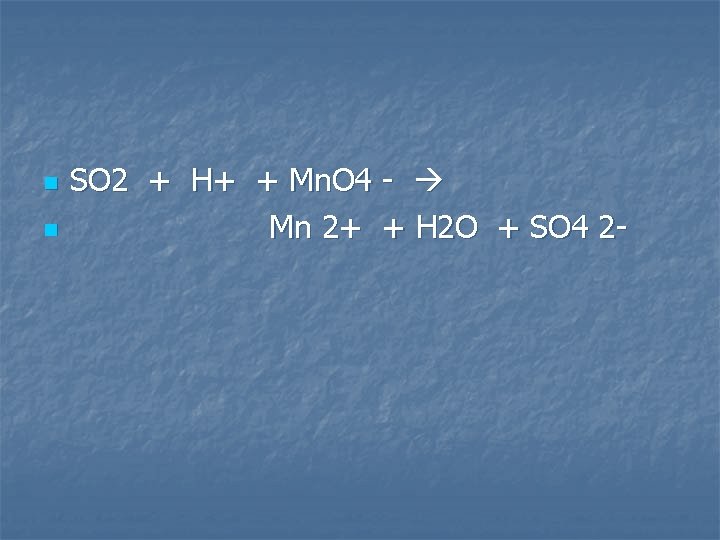
n n SO 2 + H+ + Mn. O 4 - Mn 2+ + H 2 O + SO 4 2 -

A solution containing tin (II) ions is added to an acidified solution of potassium dichromate
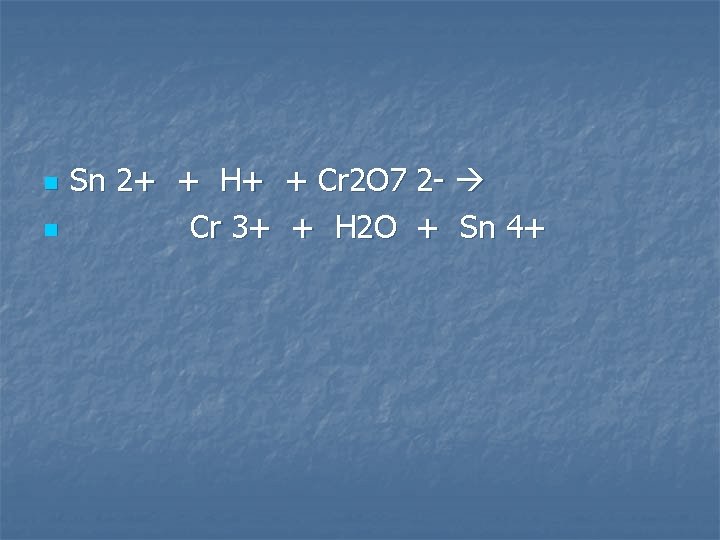
n n Sn 2+ + H+ + Cr 2 O 7 2 - Cr 3+ + H 2 O + Sn 4+

Solid silver sulfide is warmed with dilute nitric acid
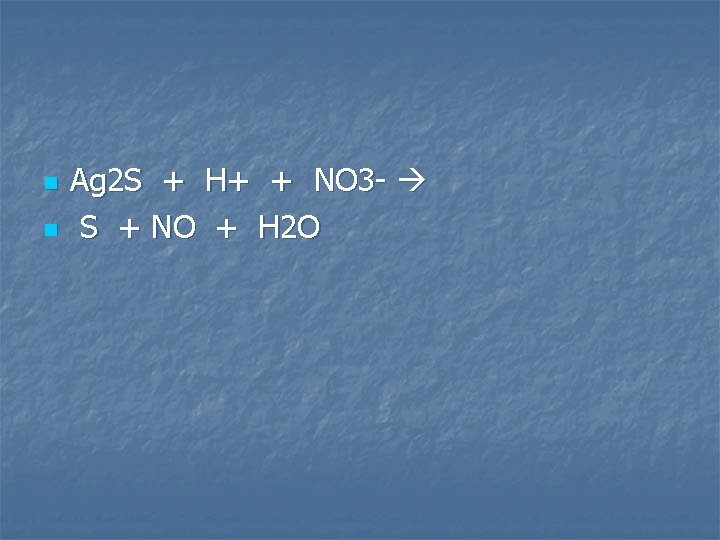
n n Ag 2 S + H+ + NO 3 - S + NO + H 2 O

Pellets of lead are dropped into hot sulfuric acid

n n Pb + H+ + SO 4 2 - H 2 O + SO 2 + Pb 2+ or Pb. SO 4

Potassium permanganate solution is added to a solution of oxalic acid, acidified with a few drops of sulfuric acid
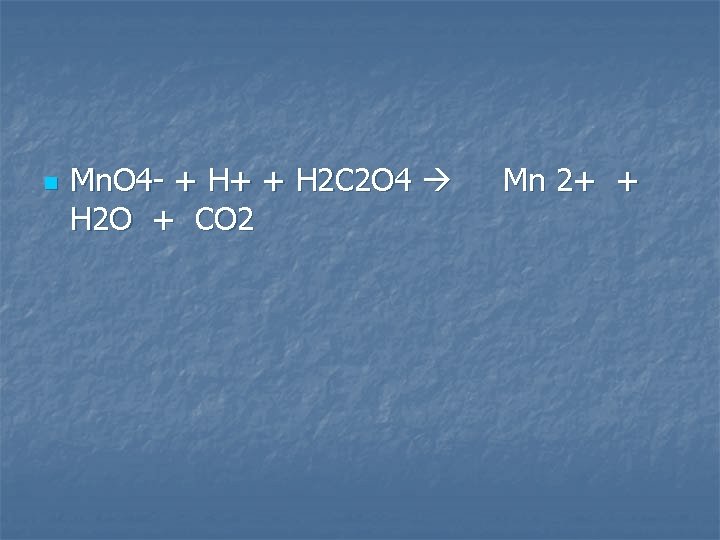
n Mn. O 4 - + H+ + H 2 C 2 O 4 H 2 O + CO 2 Mn 2+ +

Powdered iron is added to a solution of iron (III) sulfate
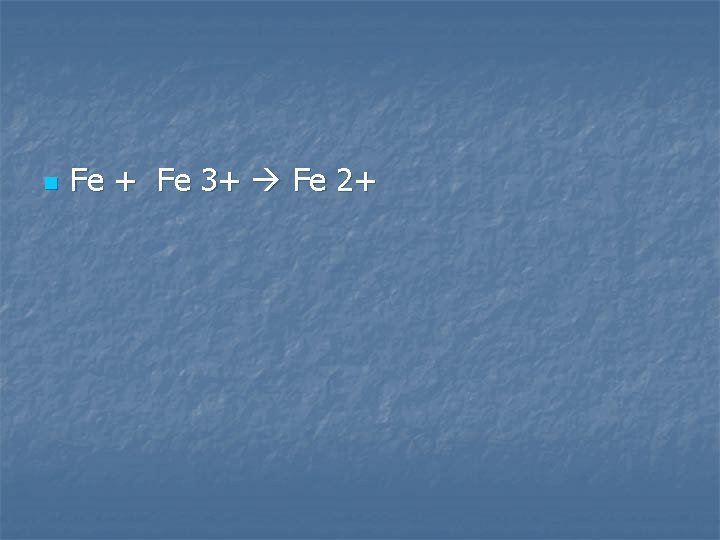
n Fe + Fe 3+ Fe 2+

A concentrated solution of hydrochloric acid is added to powdered manganese dioxide and gently heated

n H+ + Cl- + Mn. O 2 Cl 2 + Mn 2+ + H 2 O

A strip of copper metal is added to a concentrated solution of sulfuric acid
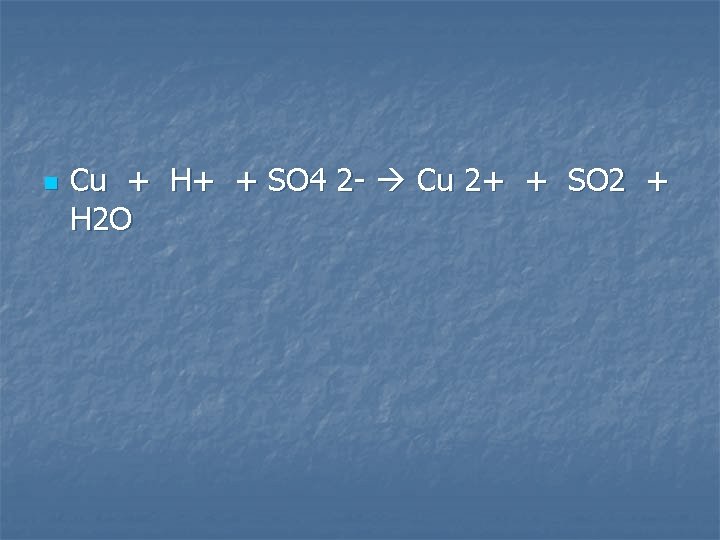
n Cu + H+ + SO 4 2 - Cu 2+ + SO 2 + H 2 O

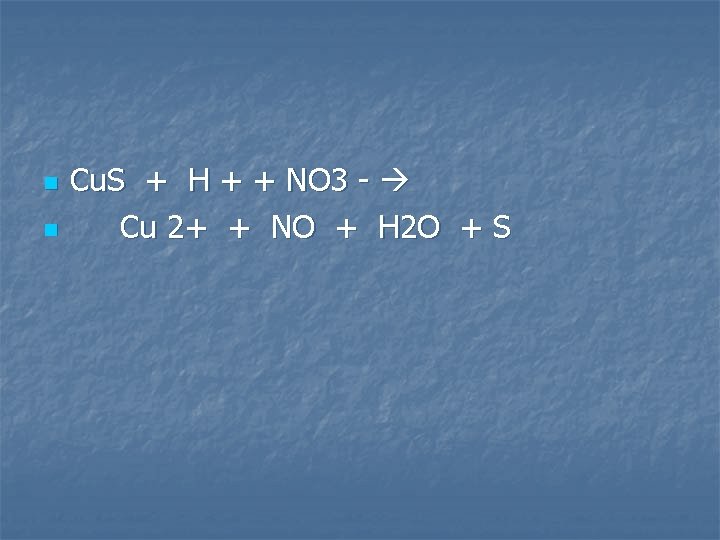
Copper (II) sulfide is oxidized by dilute nitric acid

n n Cu. S + H + + NO 3 - Cu 2+ + NO + H 2 O + S

Hydrogen peroxide solution is added to a solution of iron (II) sulfate
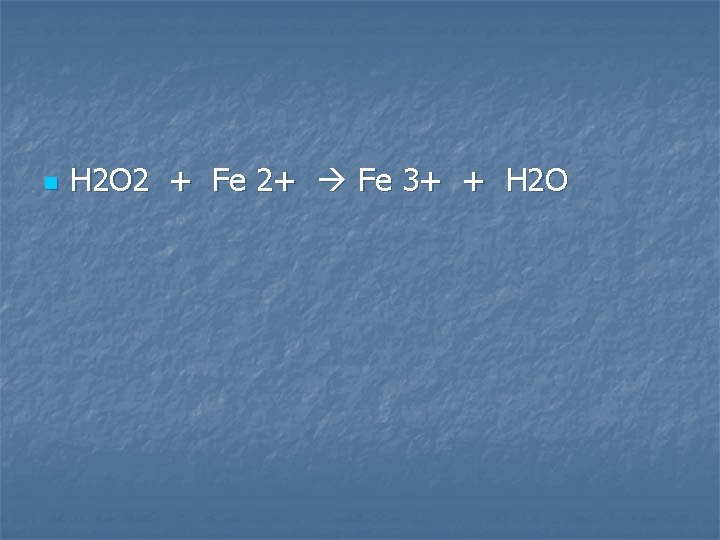
n H 2 O 2 + Fe 2+ Fe 3+ + H 2 O

Solid silver is added to a dilute nitric acid (6 M) solution
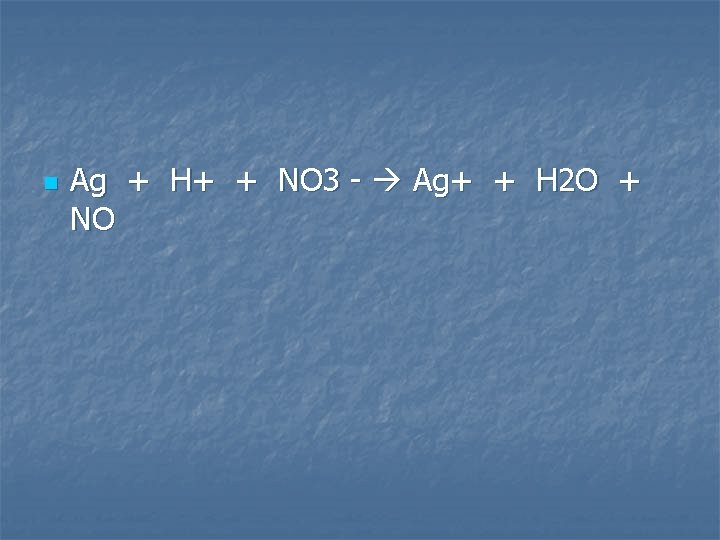
n Ag + H+ + NO 3 - Ag+ + H 2 O + NO

A solution of formic acid, HCOOH, is oxidized by an acidified solution of potassium dichromate
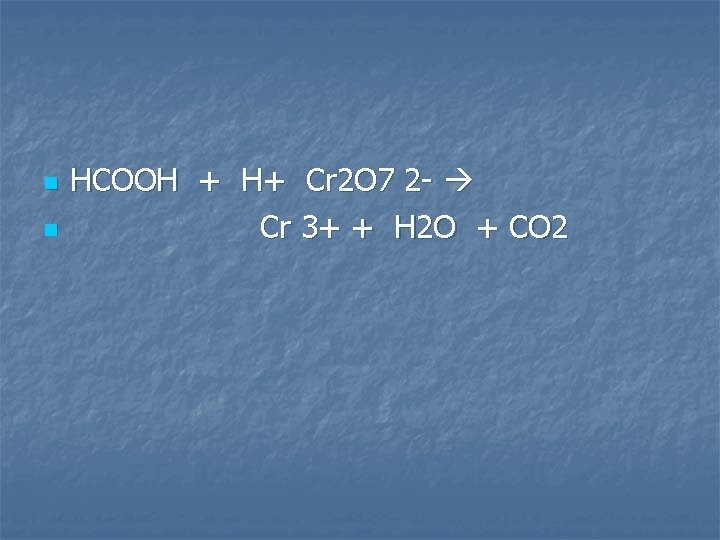
n n HCOOH + H+ Cr 2 O 7 2 - Cr 3+ + H 2 O + CO 2

A solution of tin (II) chloride is added to a solution of iron (III) sulfate
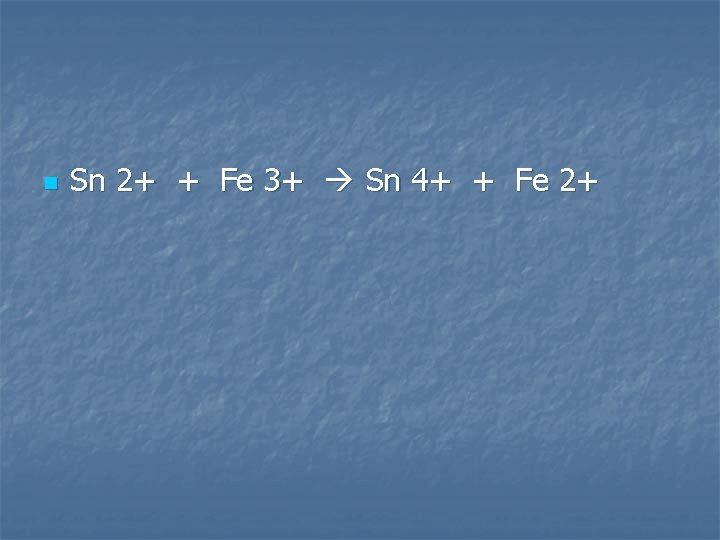
n Sn 2+ + Fe 3+ Sn 4+ + Fe 2+