Redox reactions The essence of the basic concepts

"Redox reactions. The essence of the basic concepts of redox processes: the degree of oxidation of elements in compounds, oxidant, reducing agent, oxidation and reduction processes, oxidized and reduced forms. Electronic theory of redox reactions. Redox properties of simple substances and compounds of elements depending on their position in the periodic table. The most important oxidants and reducing agents. The main types of redox reactions. The use of redox reactions in chemical analysis and drug analysis. " Lecture 9

• Man became acquainted with redox transformations and, above all, combustion in ancient times. Later - in the Bronze and Iron Ages "chemical" fire was used not only for heating homes and cooking, but also for smelting metals from ores and their processing - forging and hardening. At the same time, the corrosion processes of bronze and iron products became known. • Thus, step by step, practical experience in the use of redox transformations was accumulated. Quite a large number of them were discovered by alchemists. • Representatives of phlogiston chemistry (in the works of J. Becher, G. Stahl, K. Scheele, J. Priestley, G. Cavendish, etc. ) made a revolution in their views on redox transformations and, above all, combustion. In accordance with the views of phlogiston during the combustion of substances released elusive fiery matter (phlogiston). Then metals and other combustible bodies turn into "earth" with increasing mass. • Redox reactions play a significant role in human life, in nature and technology: respiration and metabolism, putrefaction and fermentation, fuel combustion, metal corrosion, electrolysis, metallurgy, production of inorganic and organic substances, conversion of chemical energy into electricity, etc. .
• Reactions during which the oxidation states of the elements that make up the reactants change are called redox reactions. • The degree of oxidation is the conditional charge of an atom in a molecule that would occur on an atom provided that the common electron pairs would be completely shifted to a more electronegative atom (the bond between atoms in a molecule arises due to the transition of an electron from a less electronegative to a more electronegative atom). . In simple substances, the oxidation state is always zero, because all atoms have the same electronegativity. In complex compounds, the negative degree of oxidation has elements whose electronegativity is greater, and positive - those elements whose electronegativity is less.

MAIN PROVISIONS OF THEORY OF OXIDIZING-REDUCTION PROCESSES • 1. Oxidation is the process of returning electrons by atoms, molecules, ions. • 2. Reduction is the process of receiving electrons by atoms, molecules, ions. • 3. Atoms, molecules or ions that donate electrons are called reducing agents that receive electrons - oxidants. The reducing property of an element is the ability to donate electrons, the oxidizing property is to attach them. • 4. Oxidation is always accompanied by reduction and vice versa. Reductants, giving electrons, are oxidized, and oxidants, attaching them, are reduced. Redox reactions are the unity of two opposite processes - oxidation and reduction. reductant - e → oxidant + e → reducing agent

• The redox properties of elements depend on their electronegativity: the greater the electronegativity of the element, the stronger its oxidative properties, and vice versa, the lower the electronegativity of the element, the stronger its reducing properties. The same element can exhibit different properties: oxidizing (in high oxidation states) and reducing (in lower oxidation states). Thus, sulfur in sulfuric acid, having an oxidation state of +6, exhibits oxidizing properties, and in hydrogen sulfide (oxidation state -2) - reductive. • The same element in the same oxidation state, depending on the reaction conditions, can exhibit both oxidizing and reducing properties. Thus, molecular hydrogen in reactions with metals is an oxidant, and in reactions with nonmetals or metal oxides it is a reducing agent.

THE MOST IMPORTANT OXIDIZERS AND REDUCTORS • Oxidizers. Oxidants are atoms in such oxidation states in which they are able to attach electrons, ie at high. These are neutral atoms and molecules, positive ions of metals and hydrogen, complex ions and molecules that contain elements in high oxidation states. • 1. Neutral atoms. Oxidants are molecules or atoms of elements that have near-completion external levels: s 2 p 2; s 2 p 3 s 2 p 4; s 2 p 5, ie atoms of nonmetals. Their electronegativity, and hence the oxidative properties are reduced in the following sequence: F, O, CI, N, Br, S, I, At, Se, P, Te, C, H, As, Si, B. • 2. Positive metal ions. All positive metal ions exhibit different oxidizing properties. In aqueous solutions, the oxidizing properties of these ions increase from left to right in a number of standard electrode potentials: the strongest oxidants are metal ions on the right. Thus, Cu +2 ions have sufficient oxidizing properties to oxidize iron atoms. As a result, the reaction occurs: Fe + Cu. SO 4 = Fe. SO 4 + Cu. If the metal forms ions of different charges (Fe + 2, Fe + 3), stronger oxidizing properties are shown by ions with a higher charge (Fe + 3), ie iron (III) is an ion. • 3. Positive hydrogen ions. Hydrogen ions H + contained in acid solutions, in addition to HNO 3, can oxidize metal atoms that are in a number of standard electrode potentials to hydrogen, as a result of which these metals react with acids with the release of hydrogen: Zn + 2 HC 1 = Zn. Cl 2 + H 2.
Oxidizers. • 4. Molecules and complex ions. Oxidizing properties are revealed by molecules and ions, which include elements in the highest or intermediate oxidation states. For example, the possible oxidation states of chlorine are -1, 0, +1, +3, +4, +5, +6, +7. Oxidizing properties are exhibited by compounds that contain chlorine in oxidation states greater than -1, ie from 0 to +7. Possible degrees of oxidation of manganese +2, +4, +6, +7. Oxidizing properties are exhibited by compounds containing manganese in oxidation states greater than +2, ie from +4 to +7. Manganese compounds have the strongest oxidizing properties in the oxidation state +7, for example KMn. O 4. Strong oxidizing properties are shown by concentrated sulfuric acid H 2 SO 4 (sulfur in oxidation state +6), concentrated and dilute nitric acid HNO 3 (nitrogen in oxidation state +5), potassium chromate (К 2 Сr. О 4) or dichromate (К 2 Сr 2 О 7) (chromium). . The oxidizing properties of molecules and complex ions containing elements in high oxidation states depend on the acidity of the medium. Usually these ions contain oxygen (Mn. O 4 -, Cr. O 4 2–, Cr 2 O 7 2–, SO 4 2–, etc. ). By attaching electrons, the central element (manganese, chromium, sulfur, nitrogen) reduces its oxidation state and the oxygen-containing ion is destroyed. The process involves the hydrogen ions of the medium, which bind oxygen atoms to form water molecules. As a result, the ions of the central element are released in the lower oxidation states. • Мn. О 4 - + 5 е + 8 Н + → Мn +2 + Н 2 О Сr 2 O 7 2– + 6 е + 14 Н + → 2 Сr +3 + 7 Н 2 О • 5. Anode during electrolysis. •
Reducers • Reducers are atoms or ions in oxidation states in which they are able to donate electrons, ie in lower oxidation states. These are neutral atoms or molecules, negative non-metal ions, positive metal ions in lower oxidation states, complex molecules and ions that contain elements in intermediate oxidation states, electrons (for example, at the cathode). • 1. Neutral atoms. Reducers are atoms of elements that have no more than four electrons at the external energy level, ie atoms of all metals and some nonmetals - carbon, hydrogen. The strongest reducing agents are alkali and alkaline earth metals, lanthanides and actinides. During reactions with substances in aqueous solutions, the reducing properties of metals decrease from left to right in a number of standard electrode potentials.
Reducers • 2. Negative ions of nonmetals. These ions (Cl -, Br -, I -, S – 2, N – 3) are strong reducing agents. They can donate not only the electrons, due to which they were formed, but also electrons from their external level. The stronger the oxidant is the nonmetal, the weaker the reducing properties of its negative ion. • 3. Positive metal ions. In lower oxidation states, such ions, along with oxidizing ones, exhibit reducing properties if metals can form ions with higher oxidation states: Sn + 2 Fe + 2, Cu +, Mn + 2, Cr + 3. 4. Molecules and complex ions. Elements in the intermediate oxidation states can, along with the oxidizing properties, also exhibit reducing properties, oxidizing to higher oxidation states. • 5. Cathode during electrolysis.
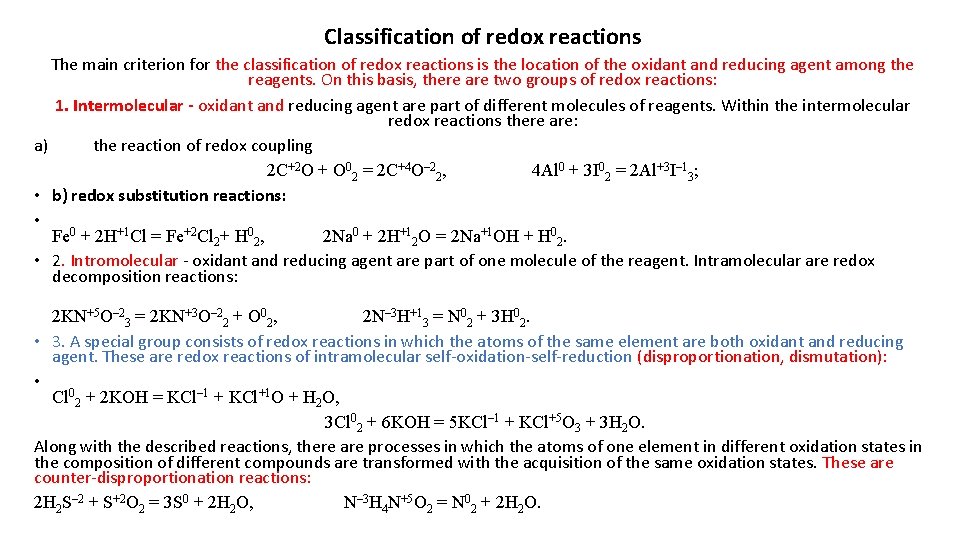
Classification of redox reactions The main criterion for the classification of redox reactions is the location of the oxidant and reducing agent among the reagents. On this basis, there are two groups of redox reactions: 1. Intermolecular - oxidant and reducing agent are part of different molecules of reagents. Within the intermolecular redox reactions there are: a) the reaction of redox coupling 2 С+2 О + О 02 = 2 С+4 О– 22, 4 Al 0 + 3 I 02 = 2 Al+3 I– 13; • b) redox substitution reactions: • Fe 0 + 2 H+1 Cl = Fe+2 Cl 2+ H 02, 2 Na 0 + 2 H+12 O = 2 Na+1 OH + H 02. • 2. Intromolecular - oxidant and reducing agent are part of one molecule of the reagent. Intramolecular are redox decomposition reactions: 2 KN+5 O– 23 = 2 KN+3 O– 22 + O 02, 2 N– 3 H+13 = N 02 + 3 H 02. • 3. A special group consists of redox reactions in which the atoms of the same element are both oxidant and reducing agent. These are redox reactions of intramolecular self-oxidation-self-reduction (disproportionation, dismutation): • Cl 02 + 2 KOH = KCl– 1 + KCl+1 O + H 2 O, 3 Cl 02 + 6 KOH = 5 KCl– 1 + KCl+5 O 3 + 3 H 2 O. Along with the described reactions, there are processes in which the atoms of one element in different oxidation states in the composition of different compounds are transformed with the acquisition of the same oxidation states. These are counter-disproportionation reactions: 2 H 2 S– 2 + S+2 O 2 = 3 S 0 + 2 H 2 O, N– 3 H 4 N+5 O 2 = N 02 + 2 H 2 O.
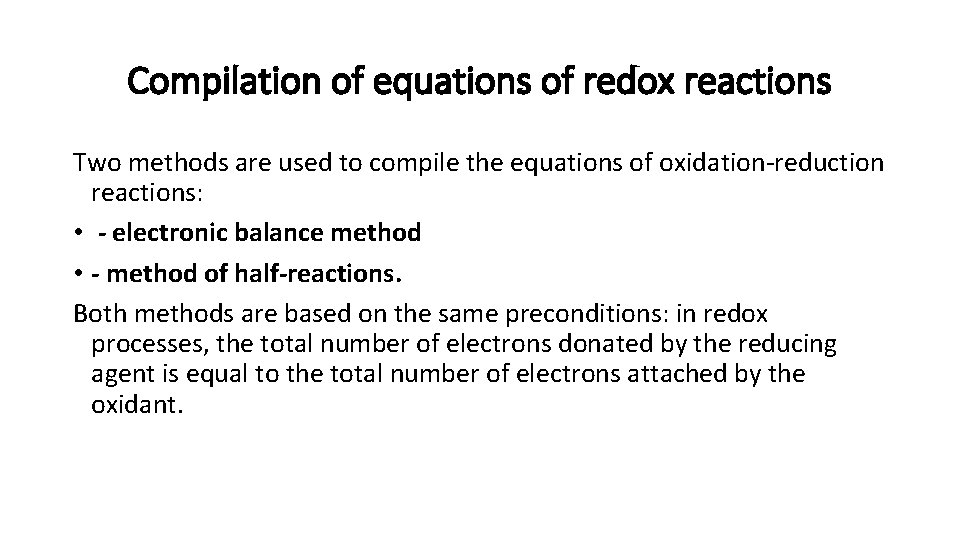
Compilation of equations of redox reactions Two methods are used to compile the equations of oxidation-reduction reactions: • - electronic balance method • - method of half-reactions. Both methods are based on the same preconditions: in redox processes, the total number of electrons donated by the reducing agent is equal to the total number of electrons attached by the oxidant.

Electronic balance method • This method is based on comparing the oxidation states of atoms in the composition of reagents and reaction products. It must be remembered that the number of electrons received by the oxidant must be equal to the number of electrons donated by the reducing agent. To compile a chemical equation, you need to know the formulas of reagents and reaction products. Example. The interaction of aluminum with a solution of hydrochloric acid occurs according to the scheme: Al + HCl → Al. Cl 3 + H 2. Determine the oxidation states of all elements: Al 0 + H+1 Cl– 1 → Al+3 Cl– 13 + H 02. The oxidation states change the Al and H atoms. During the reaction, two processes take place: 1. Oxidation Al 0 → Al + 3 2. Restoration H + 1 → H 0. Thus, Al is a reducing agent and HCl is an oxidant. The Al 0 atom is oxidized to Al + 3 giving three electrons: Al 0 - 3 e = Al + 3. The H + 1 atom is reduced to H 0 by taking one electron: H + 1 + e = H 0.
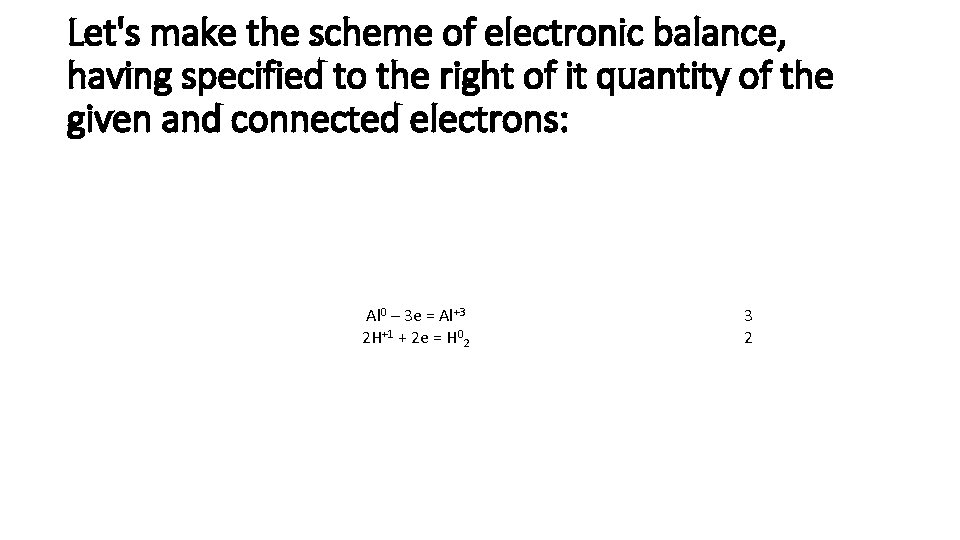
Let's make the scheme of electronic balance, having specified to the right of it quantity of the given and connected electrons: Al 0 – 3 е = Al+3 2 Н+1 + 2 е = Н 02 3 2
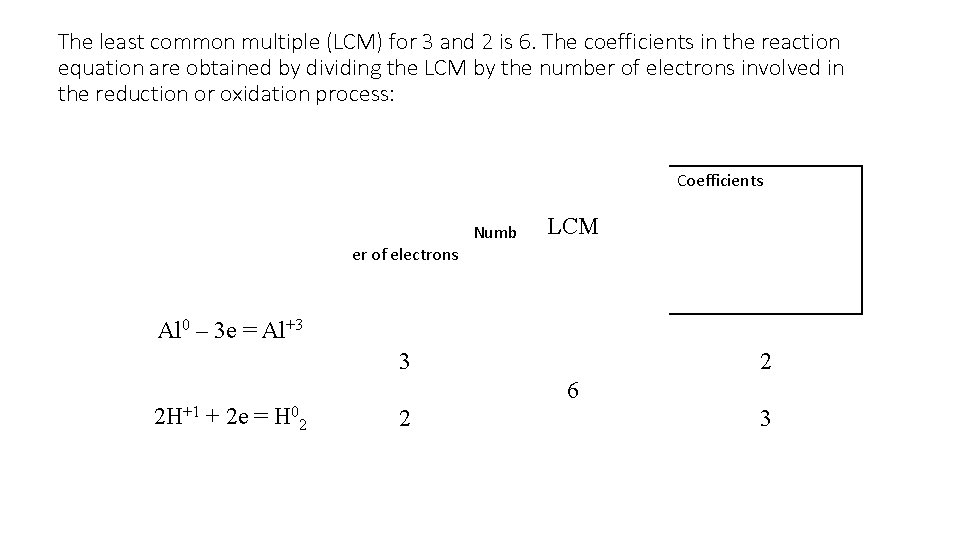
The least common multiple (LCM) for 3 and 2 is 6. The coefficients in the reaction equation are obtained by dividing the LCM by the number of electrons involved in the reduction or oxidation process: Coefficients Numb LCM er of electrons Al 0 – 3 е = Al+3 3 2 Н+1 + 2 е = Н 02 2 6 2 3
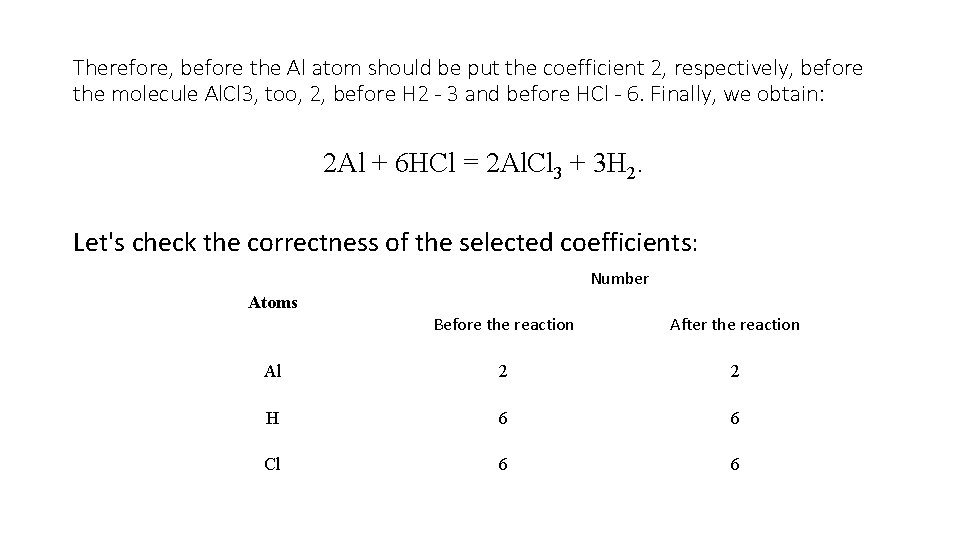
Therefore, before the Al atom should be put the coefficient 2, respectively, before the molecule Al. Cl 3, too, 2, before H 2 - 3 and before HCl - 6. Finally, we obtain: 2 Al + 6 HCl = 2 Al. Cl 3 + 3 H 2. Let's check the correctness of the selected coefficients: Number Atoms Before the reaction After the reaction Al 2 2 H 6 6 Cl 6 6
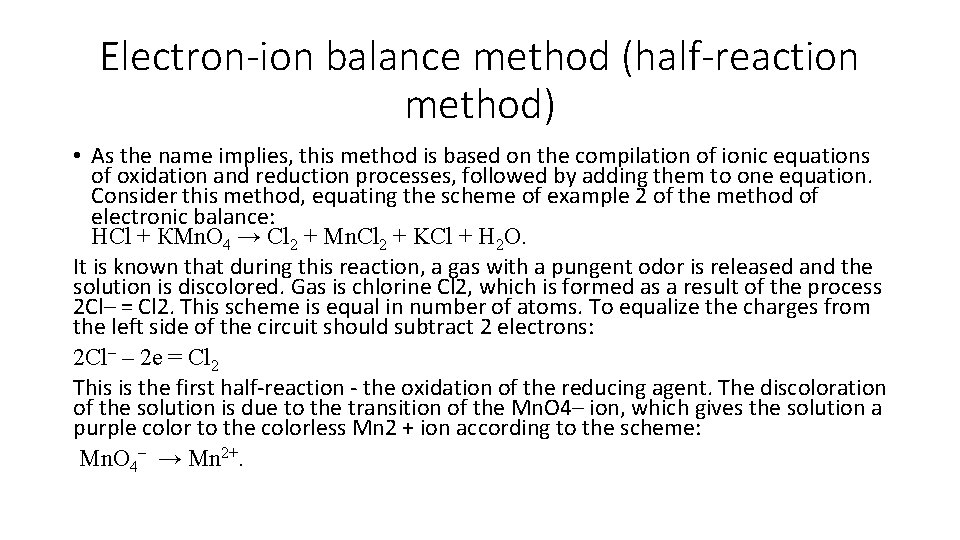
Electron-ion balance method (half-reaction method) • As the name implies, this method is based on the compilation of ionic equations of oxidation and reduction processes, followed by adding them to one equation. Consider this method, equating the scheme of example 2 of the method of electronic balance: HCl + КMn. O 4 → Cl 2 + Mn. Cl 2 + KCl + H 2 O. It is known that during this reaction, a gas with a pungent odor is released and the solution is discolored. Gas is chlorine Cl 2, which is formed as a result of the process 2 Cl– = Cl 2. This scheme is equal in number of atoms. To equalize the charges from the left side of the circuit should subtract 2 electrons: 2 Сl– – 2 е = Сl 2 This is the first half-reaction - the oxidation of the reducing agent. The discoloration of the solution is due to the transition of the Mn. O 4– ion, which gives the solution a purple color to the colorless Mn 2 + ion according to the scheme: Mn. O 4– → Mn 2+.
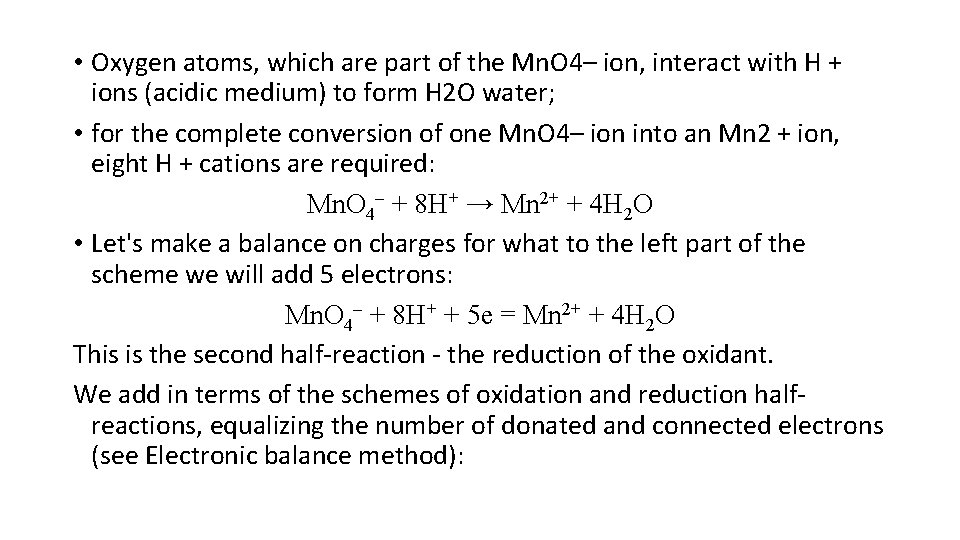
• Oxygen atoms, which are part of the Mn. O 4– ion, interact with H + ions (acidic medium) to form H 2 O water; • for the complete conversion of one Mn. O 4– ion into an Mn 2 + ion, eight H + cations are required: Mn. O 4– + 8 Н+ → Mn 2+ + 4 Н 2 О • Let's make a balance on charges for what to the left part of the scheme we will add 5 electrons: Mn. O 4– + 8 Н+ + 5 е = Mn 2+ + 4 Н 2 О This is the second half-reaction - the reduction of the oxidant. We add in terms of the schemes of oxidation and reduction halfreactions, equalizing the number of donated and connected electrons (see Electronic balance method):
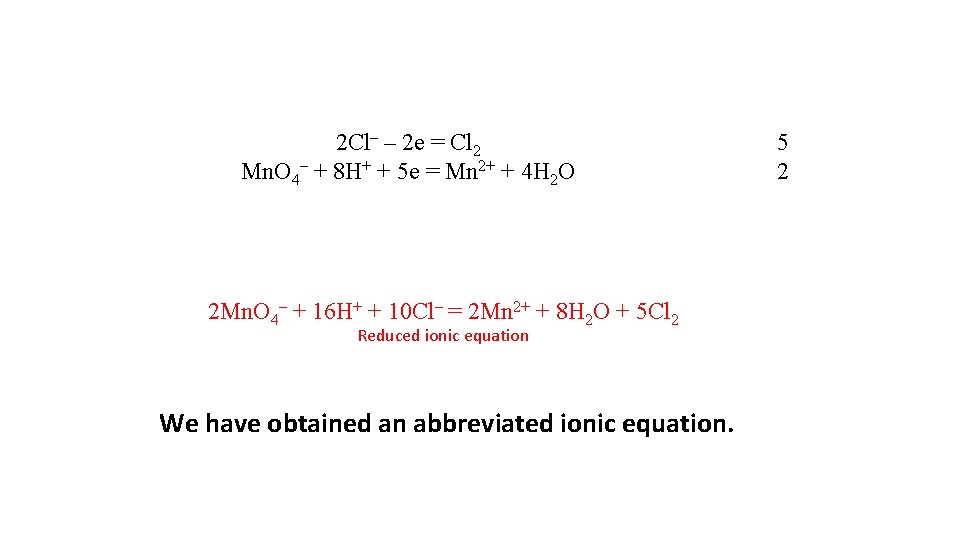
2 Сl– – 2 е = Сl 2 Mn. O 4– + 8 Н+ + 5 е = Mn 2+ + 4 Н 2 О 2 Mn. O 4– + 16 Н+ + 10 Сl– = 2 Mn 2+ + 8 Н 2 О + 5 Сl 2 Reduced ionic equation We have obtained an abbreviated ionic equation. 5 2
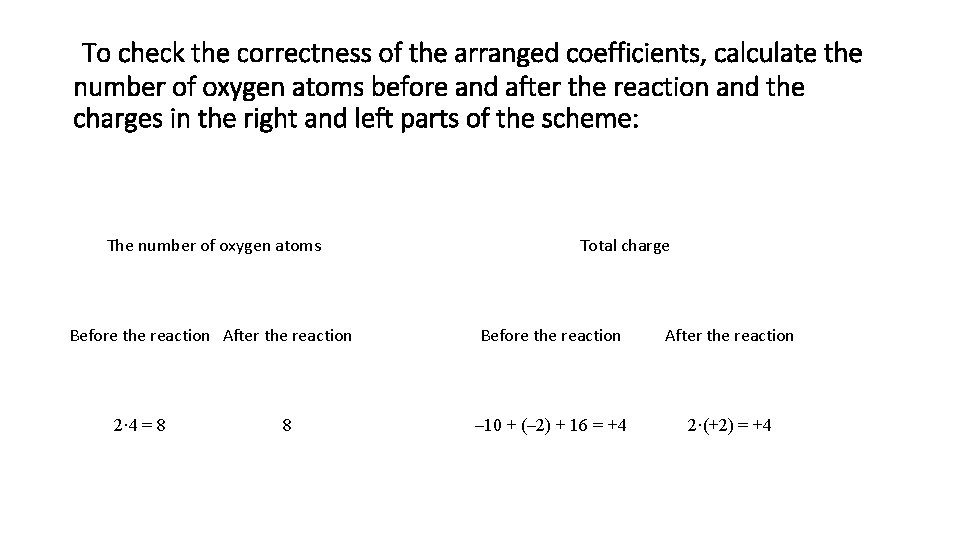
To check the correctness of the arranged coefficients, calculate the number of oxygen atoms before and after the reaction and the charges in the right and left parts of the scheme: The number of oxygen atoms Before the reaction After the reaction 2· 4 = 8 8 Total charge Before the reaction After the reaction – 10 + (– 2) + 16 = +4 2·(+2) = +4

• The main advantages of the method of electron-ion balance (half-reaction) are: Use in the process of equalization of real ions. - It is not necessary to determine the oxidation states of the elements before and after the reaction. - It is not necessary to know all the reaction products (they are determined from the halfreactions). - The role of the environment is clearly visible. When compiling the equations of half-reactions, there is always a need to balance the amounts of oxygen and hydrogen atoms in the left and right parts of each half-reaction. There a number of rules for this: • 1. To balance the number of oxygen atoms in an acidic environment, the required number is introduced with water molecules, adding on the other side of the reaction twice the number of H + cations. • 2. In an alkaline medium, oxygen atoms are introduced by adding twice the number of OH– anions on the desired side of the equation, and twice as many H 2 O molecules on the opposite side. • 3. The required number of hydrogen atoms in an acidic environment is introduced with H + ions. In alkaline - H 2 O molecules, adding the same number of OH– anions on the opposite side. • 4. Note that the method of half-reactions (ion-electron balance) is suitable only for equalization of the equations of reactions occurring in the aqueous medium. Electronic balance method - for any medium and physical state (solid, gaseous, melt, etc. ).
- Slides: 20