Redox Reactions Section 19 1 Oxidation and Reduction
















- Slides: 38


Redox Reactions Section 19. 1 Oxidation and Reduction Section 19. 2 Balancing Redox Equations Click a hyperlink or folder tab to view the corresponding slides. Exit

Section 19. 1 Oxidation and Reduction • Describe the processes of oxidation and reduction. • Identify oxidizing and reducing agents. • Determine the oxidation number of an element in a compound. • Interpret redox reactions in terms of change in oxidation state. spectator ion: an ion that does not participate in a reaction and is not usually shown in an ionic equation

Section 19. 1 Oxidation and Reduction (cont. ) oxidation-reduction reaction reduction redox reaction oxidizing agent oxidation reducing agent Oxidation and reduction are complementary—as an atom is oxidized, another atom is reduced.

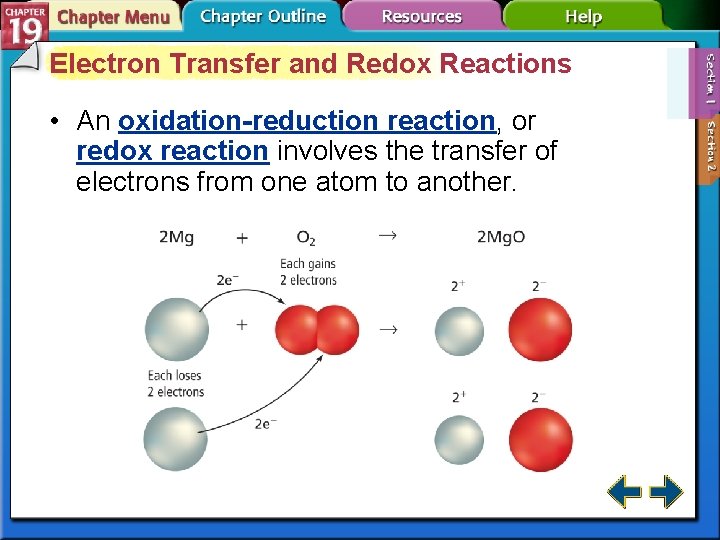
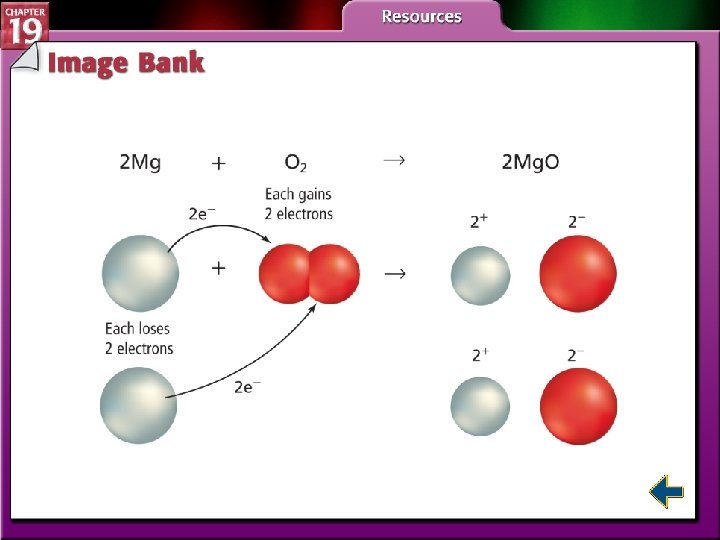
Electron Transfer and Redox Reactions • An oxidation-reduction reaction, or redox reaction involves the transfer of electrons from one atom to another.

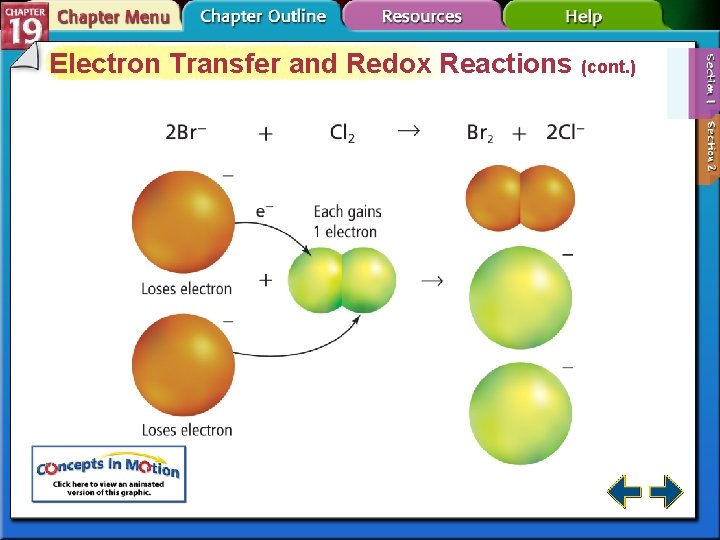
Electron Transfer and Redox Reactions (cont. )

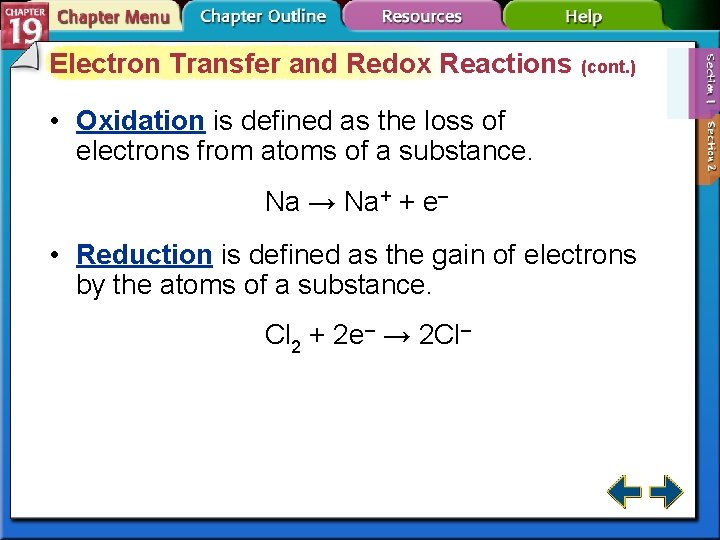
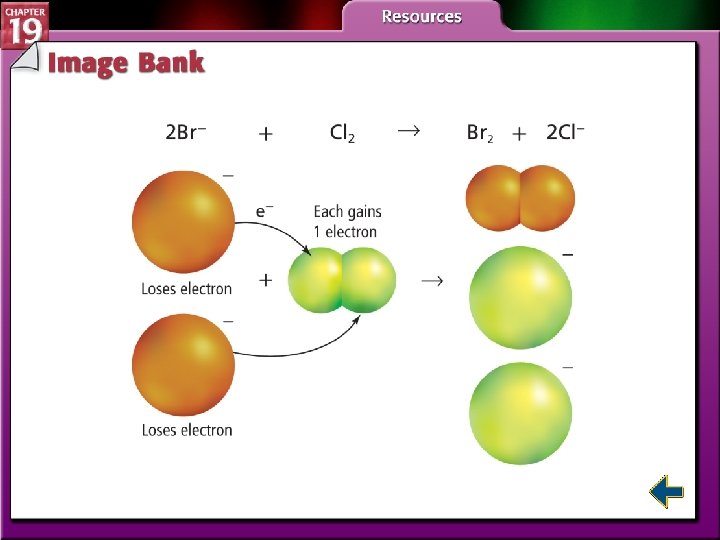
Electron Transfer and Redox Reactions (cont. ) • Oxidation is defined as the loss of electrons from atoms of a substance. Na → Na+ + e– • Reduction is defined as the gain of electrons by the atoms of a substance. Cl 2 + 2 e– → 2 Cl–

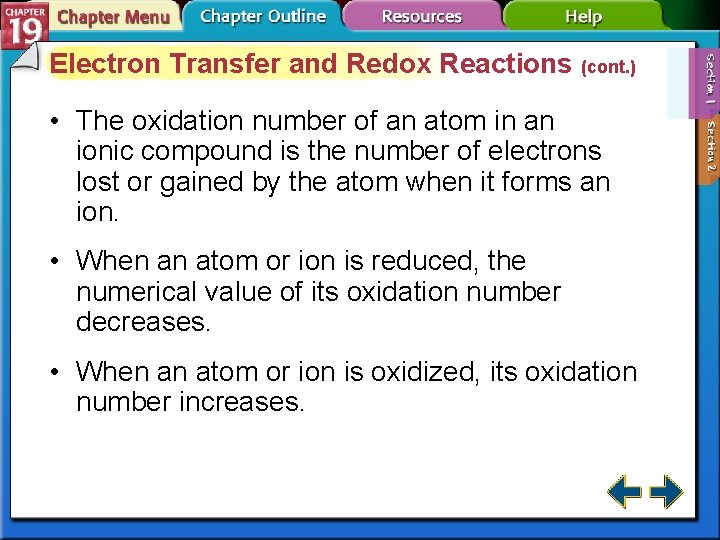
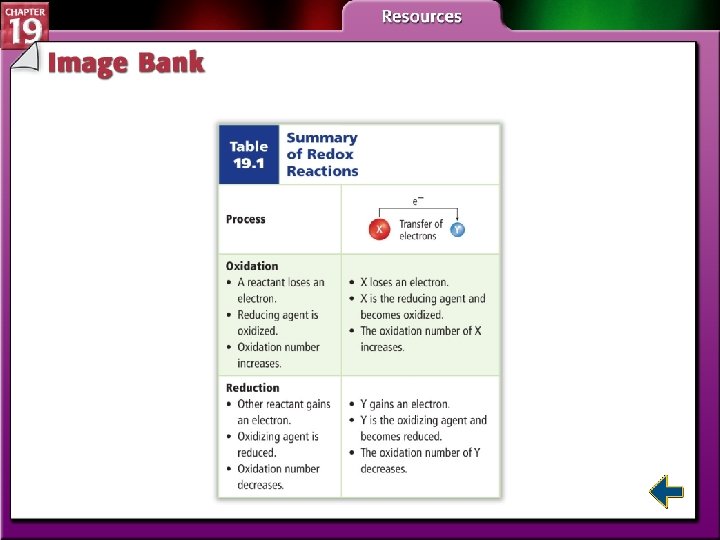
Electron Transfer and Redox Reactions (cont. ) • The oxidation number of an atom in an ionic compound is the number of electrons lost or gained by the atom when it forms an ion. • When an atom or ion is reduced, the numerical value of its oxidation number decreases. • When an atom or ion is oxidized, its oxidation number increases.

Electron Transfer and Redox Reactions (cont. ) • Oxidation numbers are tools that scientists use to keep track of the movement of electrons in a redox reaction.

Oxidizing and Reducing Agents • The substance that oxidizes another substance by accepting its electrons is called an oxidizing agent. • The oxidizing agent is the substance that is reduced in a redox reaction.

Oxidizing and Reducing Agents (cont. ) • The substance that reduces another substance by losing its electrons is the reducing agent. • The reducing agent is the substance that is oxidized in a redox reaction.

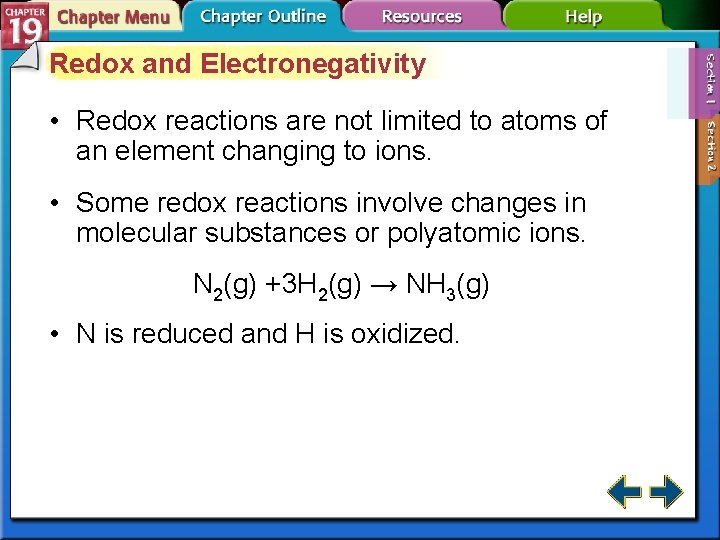
Redox and Electronegativity • Redox reactions are not limited to atoms of an element changing to ions. • Some redox reactions involve changes in molecular substances or polyatomic ions. N 2(g) +3 H 2(g) → NH 3(g) • N is reduced and H is oxidized.

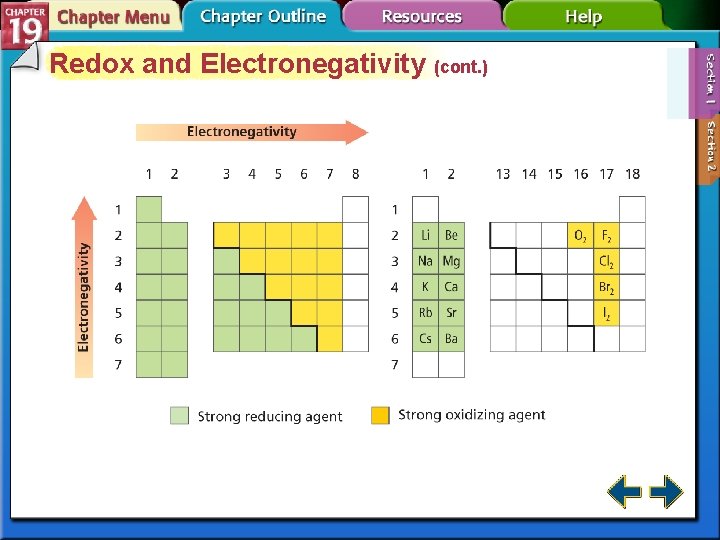
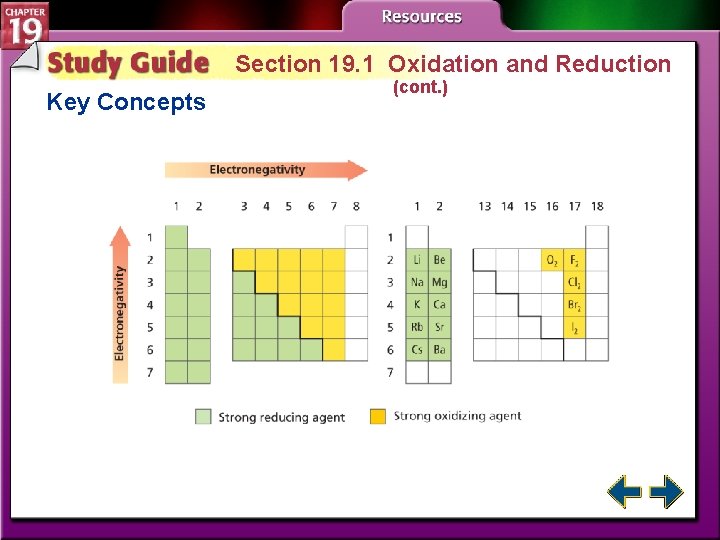
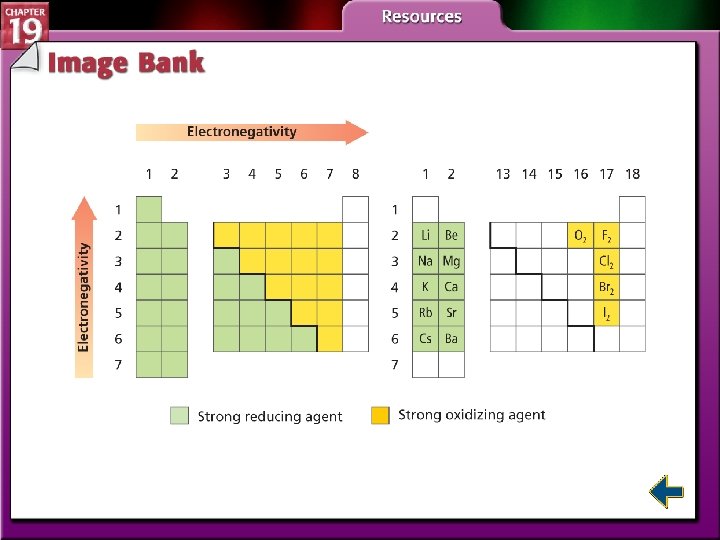
Redox and Electronegativity (cont. ) • To determine which was oxidized and which was reduced, you must know which atom is more electronegative. • Elements with high electronegativity are strong oxidizing agents.

Redox and Electronegativity (cont. )

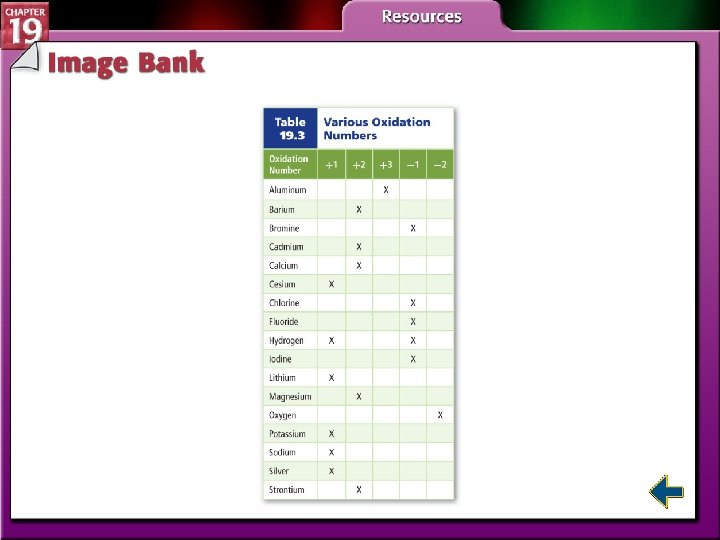
Determining Oxidation Numbers • To understand all types of redox reactions, the oxidation number of the atoms involved in the reaction must be determined.

Determining Oxidation Numbers (cont. )

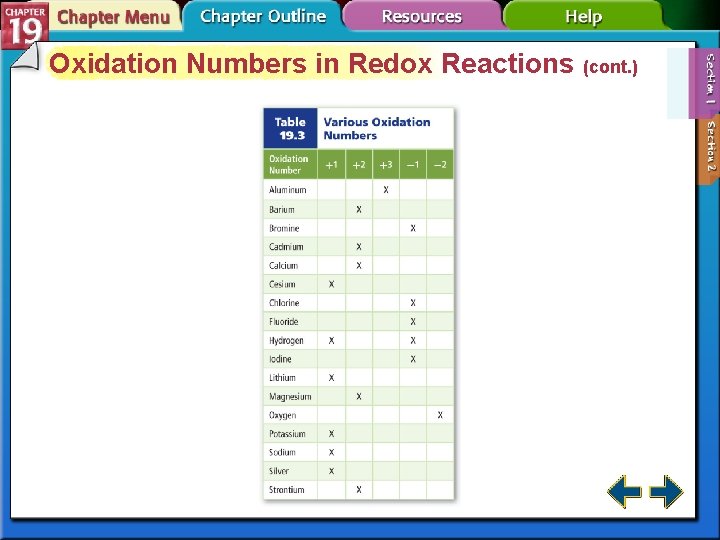
Oxidation Numbers in Redox Reactions • Oxidation-reduction reactions are changes in oxidation number. • Atoms that are reduced have their oxidation number decreased. • Atoms that are oxidized have their oxidation number increased.

Oxidation Numbers in Redox Reactions (cont. )

Section 19. 2 Balancing Redox Reactions • Relate changes in oxidation number to the transfer of electrons. • Use changes in oxidation number to balance redox equations. • Balance net ionic redox equations using the oxidation-number method. net ionic equation: an ionic equation that includes only the particles that participate in the reaction

Section 19. 2 Balancing Redox Reactions (cont. ) oxidation-number method species half-reaction Redox equations are balanced when the total increase in oxidation numbers equals the total decrease in oxidation numbers of the atoms involved in the reaction.

The Oxidation-Number Method • Chemical equations must be balanced to show the correct quantities of reactants and products. • The number of electrons transferred from atoms must equal the number of electrons accepted by other atoms.

The Oxidation-Number Method (cont. ) • The total increase in oxidation numbers must equal the total decrease in oxidation numbers in the reaction. • This method is called the oxidation number method.

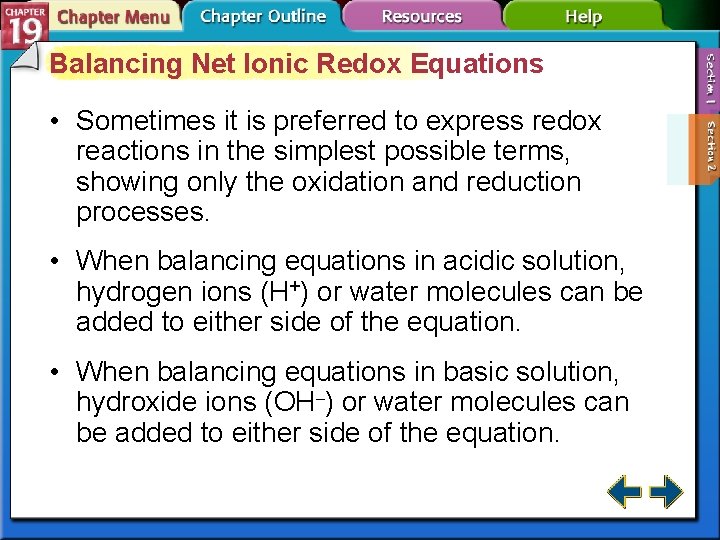
Balancing Net Ionic Redox Equations • Sometimes it is preferred to express redox reactions in the simplest possible terms, showing only the oxidation and reduction processes. • When balancing equations in acidic solution, hydrogen ions (H+) or water molecules can be added to either side of the equation. • When balancing equations in basic solution, hydroxide ions (OH–) or water molecules can be added to either side of the equation.

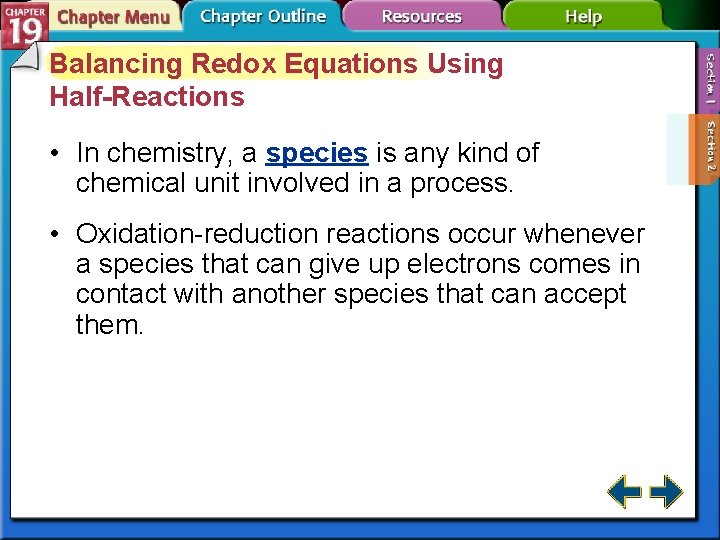
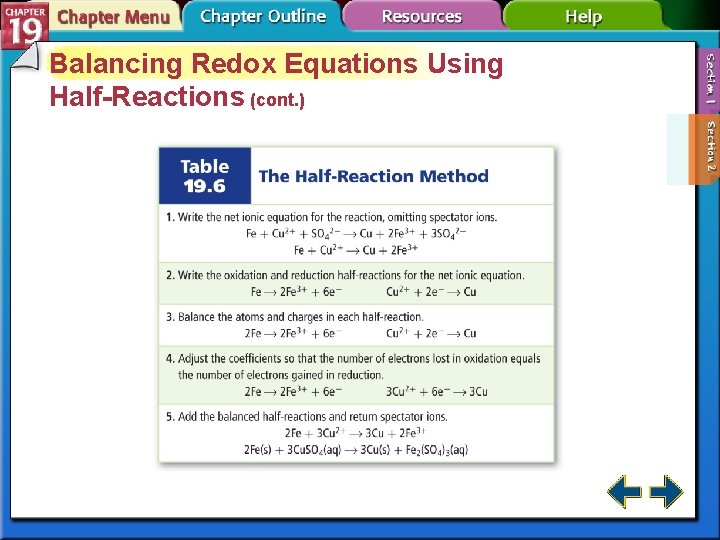
Balancing Redox Equations Using Half-Reactions • In chemistry, a species is any kind of chemical unit involved in a process. • Oxidation-reduction reactions occur whenever a species that can give up electrons comes in contact with another species that can accept them.

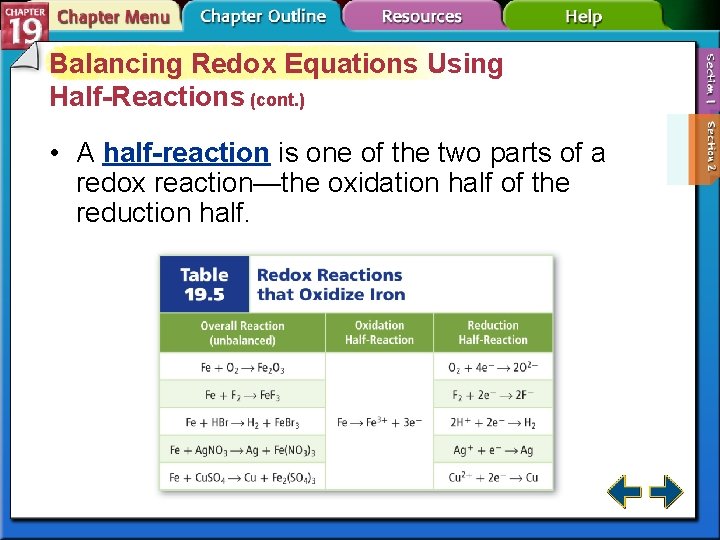
Balancing Redox Equations Using Half-Reactions (cont. ) • A half-reaction is one of the two parts of a redox reaction—the oxidation half of the reduction half.

Balancing Redox Equations Using Half-Reactions (cont. )

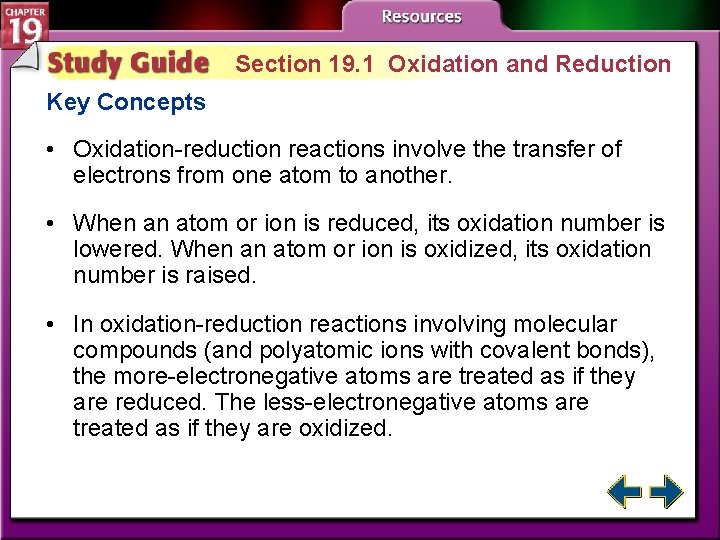
Section 19. 1 Oxidation and Reduction Key Concepts • Oxidation-reduction reactions involve the transfer of electrons from one atom to another. • When an atom or ion is reduced, its oxidation number is lowered. When an atom or ion is oxidized, its oxidation number is raised. • In oxidation-reduction reactions involving molecular compounds (and polyatomic ions with covalent bonds), the more-electronegative atoms are treated as if they are reduced. The less-electronegative atoms are treated as if they are oxidized.

Section 19. 1 Oxidation and Reduction Key Concepts (cont. )

Click on an image to enlarge.








