Redox Reactions Reduction Oxidation What is a redox













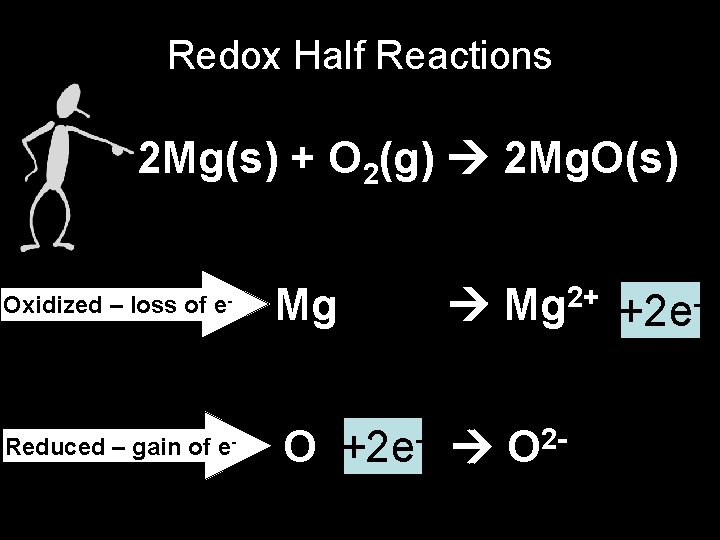
- Slides: 22

Redox Reactions Reduction Oxidation

What is a redox reaciton? • Redox (or oxidation-reduction) reactions are reactions in which electrons are transferred from one atom to another. • And redox reactions are…EVERYWHERE!

For Example… • Zinc, a component of galvanized steel, can react with oxygen in the following reaction: • 2 Zn (s) + O 2 (g) 2 Zn. O (s) • But really, you can also think of it like this… • 2 Zn (s) + O 2 (g) 2 Zn 2+ (s) + 2 O 2 - (s) • As you can see…Zinc loses electrons while oxygen gains electrons.

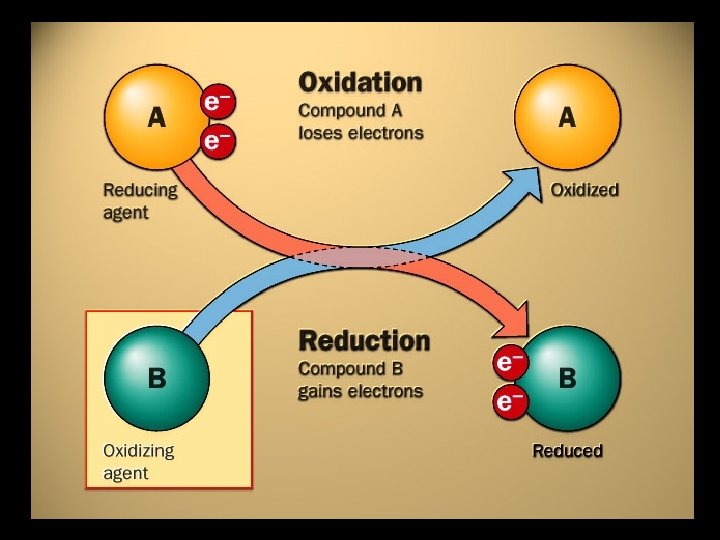
It’s actually TWO reactions! • Oxidation reaction – a reaction in which an element loses electrons Zn [Zn]2+ + 2 e • Reduction reaction – a reaction in which an element gains electrons O + 2 e- [O]2 -


Just Remember… O xidation I s L oss R eduction I s G ain

And don’t forget… This is all based on the Octet Rule!

Oxidation Numbers • In order to determine which atoms are reduced and which are oxidzed, we must assign each element an oxidation number • Remember! Oxidation numbers are… • The hypothetical electrical charge on an element or ion

` Rules for Assigning Oxidation Numbers

A Few Basics… • The oxidation number of Oxygen is always -2 (with the exception of peroxides where Oxygen is -1) • The oxidation number of Hydrogen is always +1 (except when combined to form metal hydrides where it is -1) • Oxidation numbers for elements of very high and very low electronegativity are based on the periodic table.

Oxidation Numbers 1. The oxidation number of atoms in their elemental state is zero. F -1 O -2 Oxidation state of O in O 2? H +1 0 Cl -1

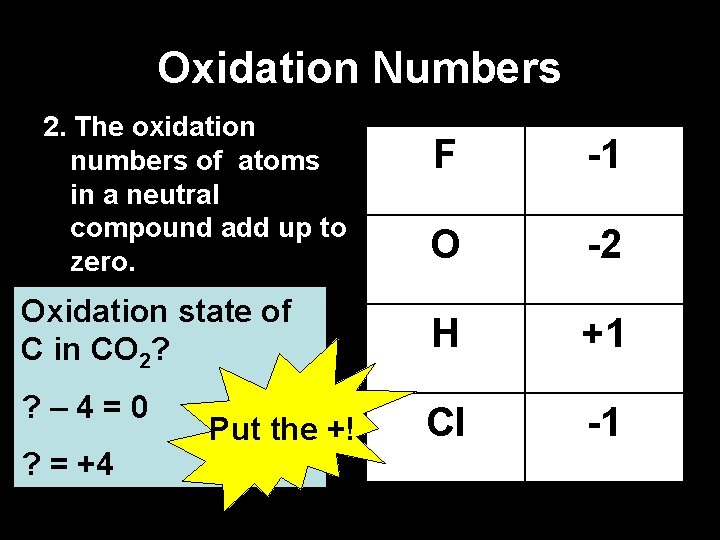
Oxidation Numbers 2. The oxidation numbers of atoms in a neutral compound add up to zero. F -1 O -2 Oxidation state of C in CO 2? H +1 ? – 4=0 Cl -1 ? = +4 Put the +!

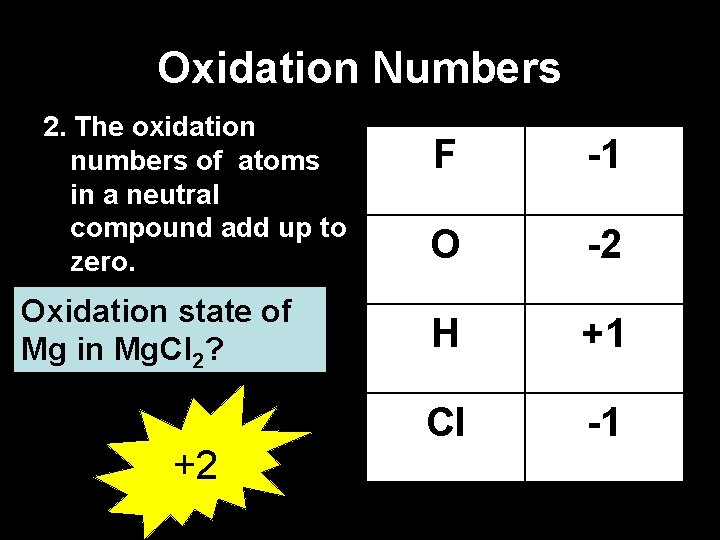
Oxidation Numbers 2. The oxidation numbers of atoms in a neutral compound add up to zero. Oxidation state of Mg in Mg. Cl 2? +2 F -1 O -2 H +1 Cl -1

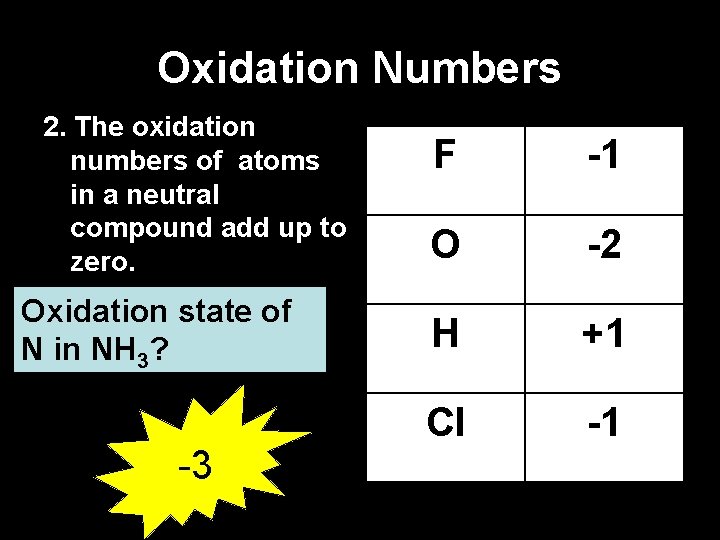
Oxidation Numbers 2. The oxidation numbers of atoms in a neutral compound add up to zero. Oxidation state of N in NH 3? -3 F -1 O -2 H +1 Cl -1

Oxidation Numbers 3. The oxidation numbers of a monatomic ion is equal to its charge. Oxidation state of S in S 2 -? -2 F -1 O -2 H +1 Cl -1

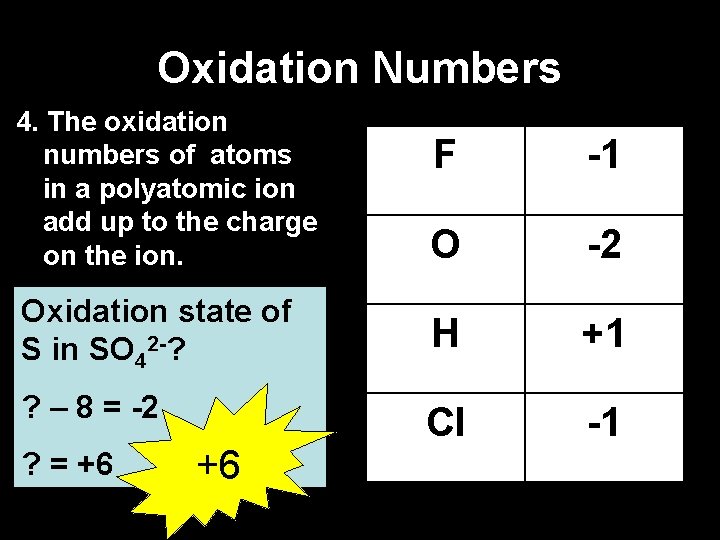
Oxidation Numbers 4. The oxidation numbers of atoms in a polyatomic ion add up to the charge on the ion. F -1 O -2 Oxidation state of S in SO 42 -? H +1 ? – 8 = -2 Cl -1 ? = +6 +6

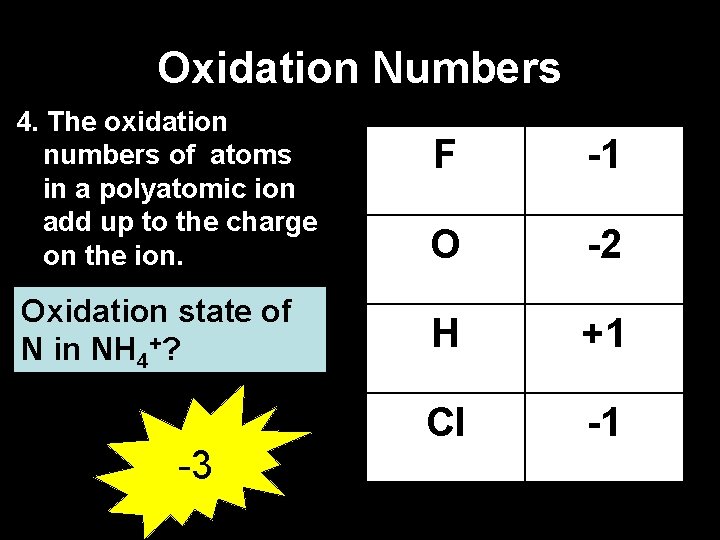
Oxidation Numbers 4. The oxidation numbers of atoms in a polyatomic ion add up to the charge on the ion. Oxidation state of N in NH 4+? -3 F -1 O -2 H +1 Cl -1

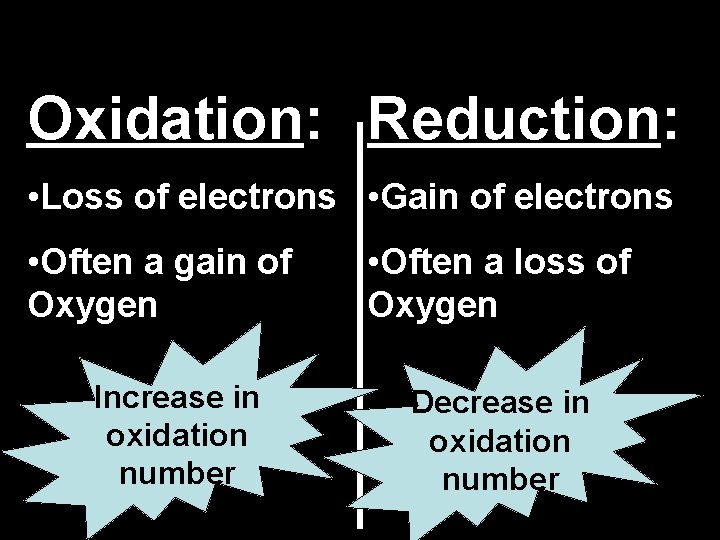
Oxidation: Reduction: • Loss of electrons • Gain of electrons • Often a gain of Oxygen Increase in oxidation number • Often a loss of Oxygen Decrease in oxidation number

For these two reactions… 1. Burning magnesium 2. Copper in silver nitrate solution Write… • Word equation • Balanced chemical equation

Redox Half Reactions We can separate redox reactions into half reactions. These reactions illustrate the flow of electrons from the electron donor (reducing agent) to the electron acceptor (oxidizing agent).
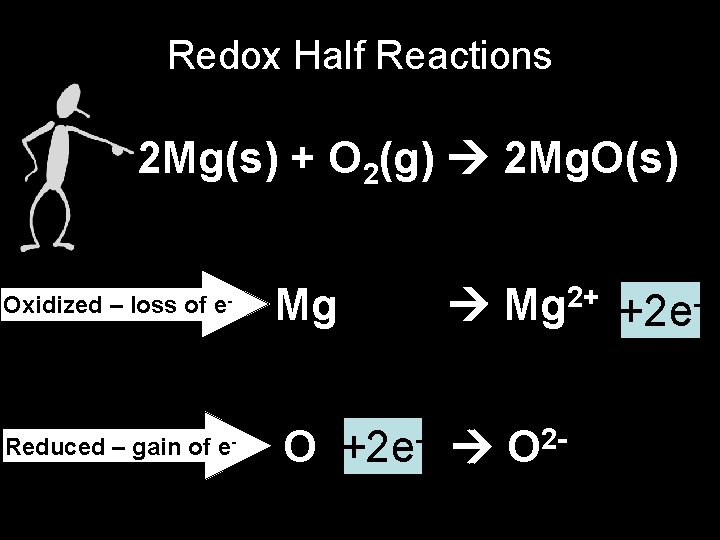
Redox Half Reactions 2 Mg(s) + O 2(g) 2 Mg. O(s) Oxidized – loss of e- Reduced – gain of e- Mg 2+ +2 e- O +2 e- O 2 -

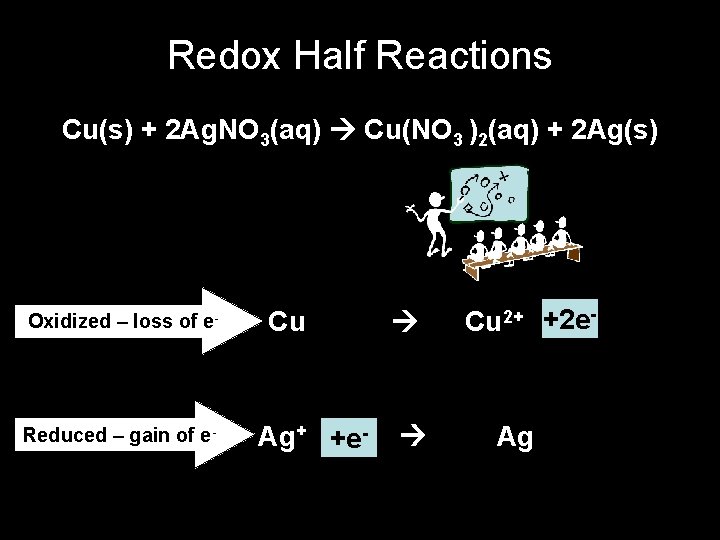
Redox Half Reactions Cu(s) + 2 Ag. NO 3(aq) Cu(NO 3 )2(aq) + 2 Ag(s) Oxidized – loss of e- Reduced – gain of e- Cu Ag+ +e- Cu 2+ +2 e- Ag