Redox Reactions Reduction Oxidation Oxidation Reduction Gain of




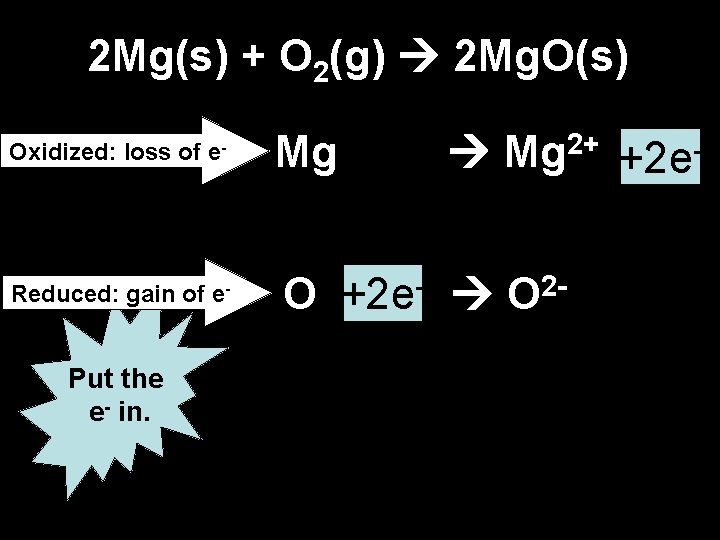






- Slides: 23

Redox Reactions. Reduction Oxidation

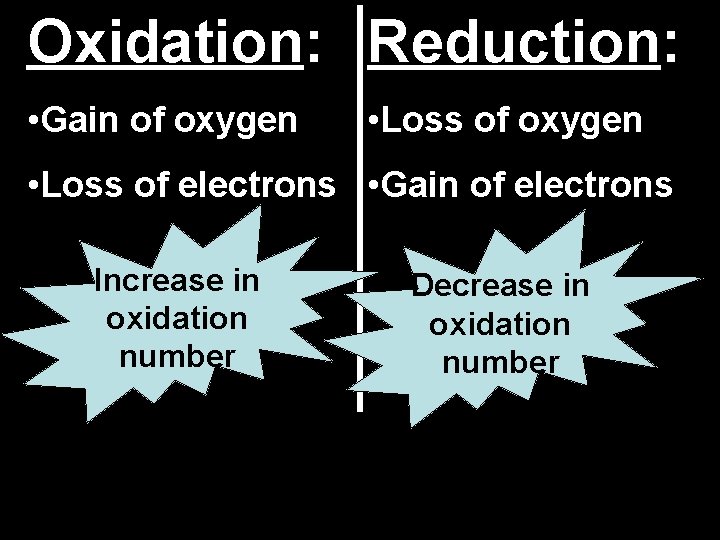
Oxidation: Reduction: • Gain of oxygen • Loss of electrons • Gain of electrons Increase in oxidation number Decrease in oxidation number

LEO “GER” SAYS ________ L = Losing E = Electrons O = Oxidation G = Gaining E = Electrons R = Reduction

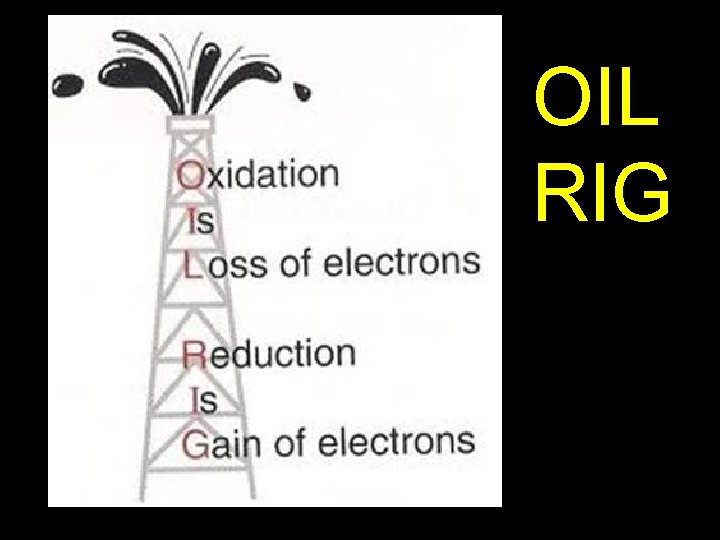
OIL RIG

Oxidation Numbers Rules (Look at your rule list too) The following is not a complete list of rules. • The oxidation number of an atom in an element is zero. E. g. Mg in Mg, O in O 2.

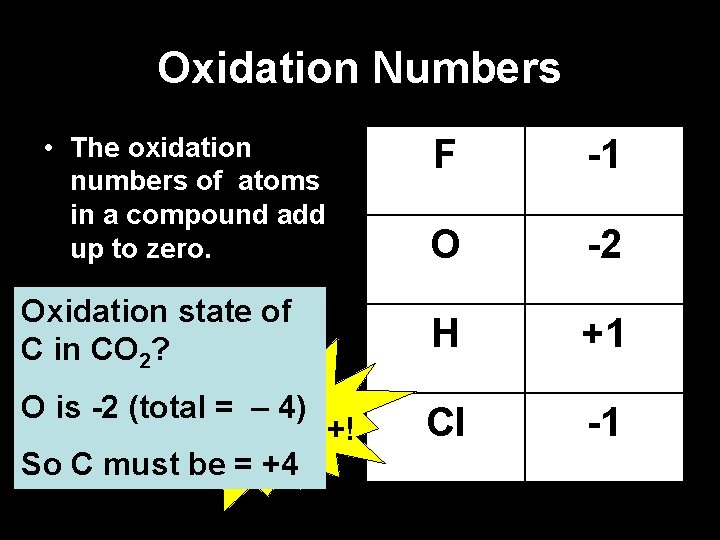
Oxidation Numbers • The oxidation numbers of atoms in a compound add up to zero. F -1 O -2 Oxidation state of C in CO 2? H +1 O is -2 (total = – 4) Put the +! So C must be = +4 Cl -1

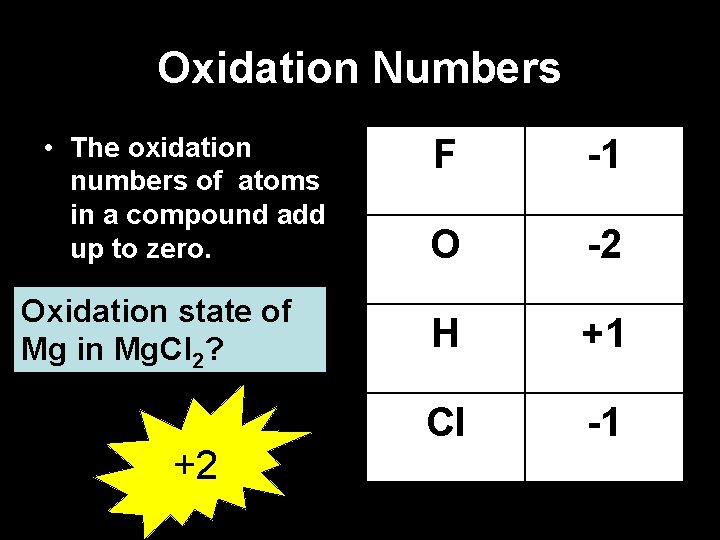
Oxidation Numbers • The oxidation numbers of atoms in a compound add up to zero. Oxidation state of Mg in Mg. Cl 2? +2 F -1 O -2 H +1 Cl -1

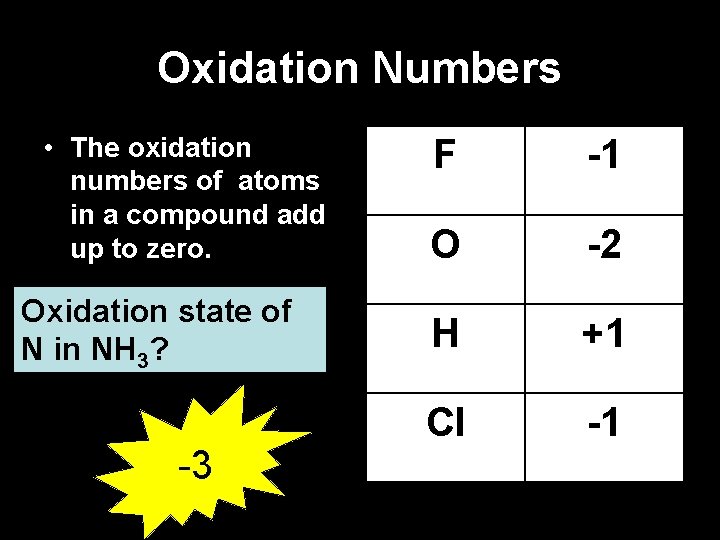
Oxidation Numbers • The oxidation numbers of atoms in a compound add up to zero. Oxidation state of N in NH 3? -3 F -1 O -2 H +1 Cl -1

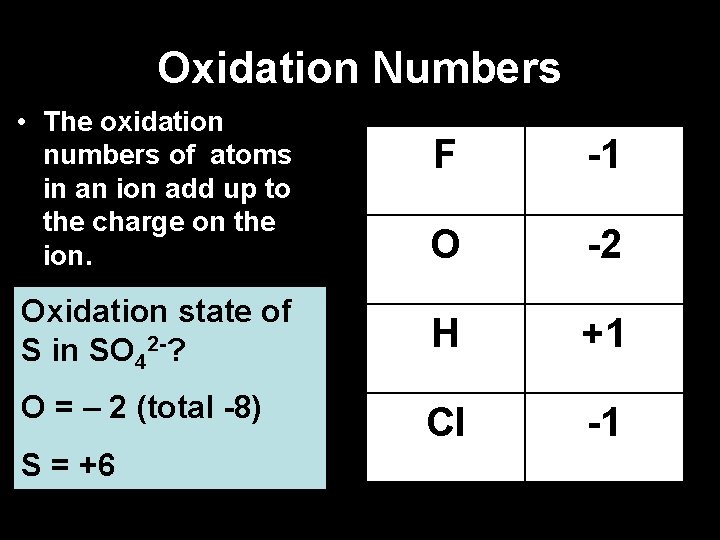
Oxidation Numbers • The oxidation numbers of atoms in an ion add up to the charge on the ion. F -1 O -2 Oxidation state of S in SO 42 -? H +1 O = – 2 (total -8) Cl -1 S: = +6 ? ? ? X - 8 = -2 S

2 Experiments: 1. Burning magnesium 2. Copper in silver nitrate solution • Word equation • Balanced symbol equation
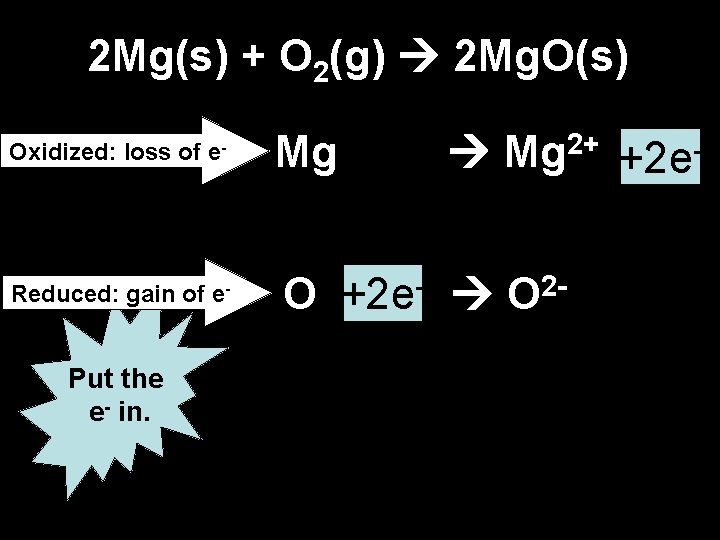
2 Mg(s) + O 2(g) 2 Mg. O(s) Oxidized: loss of e- Reduced: gain of e- Put the e- in. Mg O Mg 2+ +2 e+2 e O 2 -

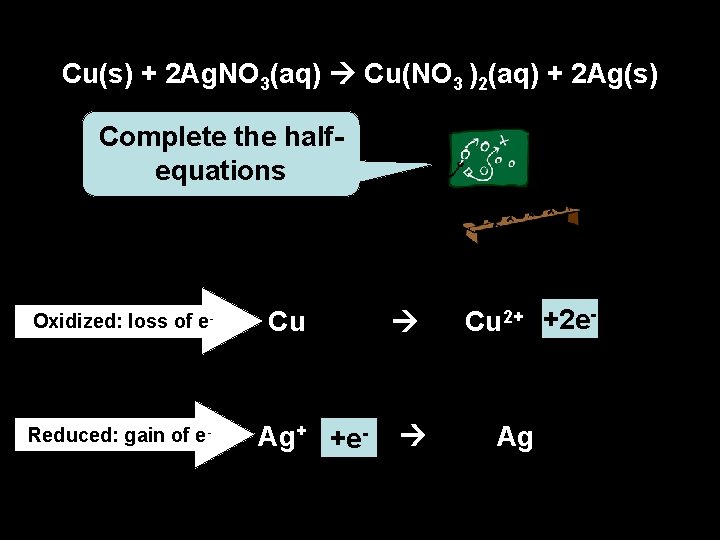
Cu(s) + 2 Ag. NO 3(aq) Cu(NO 3 )2(aq) + 2 Ag(s) Complete the halfequations Oxidized: loss of e- Reduced: gain of e- Cu Ag+ +e- Cu 2+ +2 e- Ag

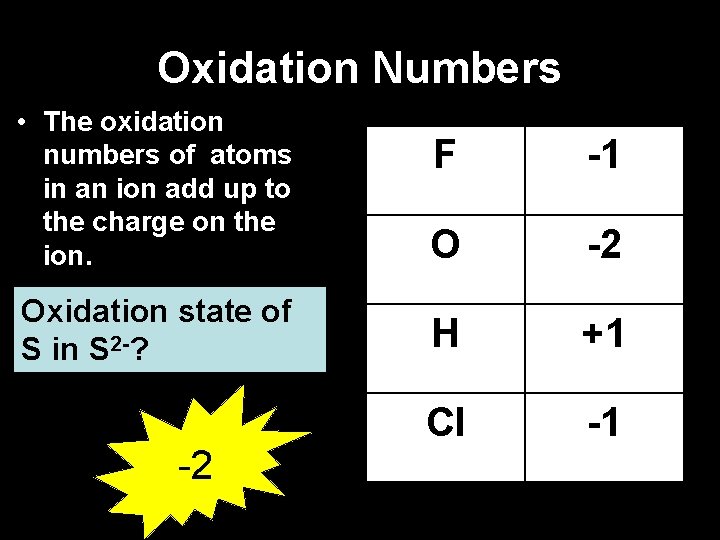
Oxidation Numbers • The oxidation numbers of atoms in an ion add up to the charge on the ion. F -1 O -2 Oxidation state of S in S 2 -? H +1 Cl -1 -2

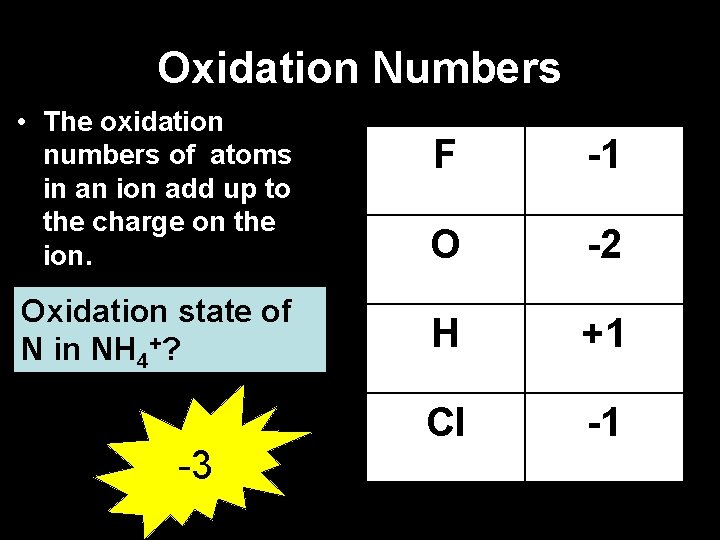
Oxidation Numbers • The oxidation numbers of atoms in an ion add up to the charge on the ion. Oxidation state of N in NH 4+? -3 F -1 O -2 H +1 Cl -1

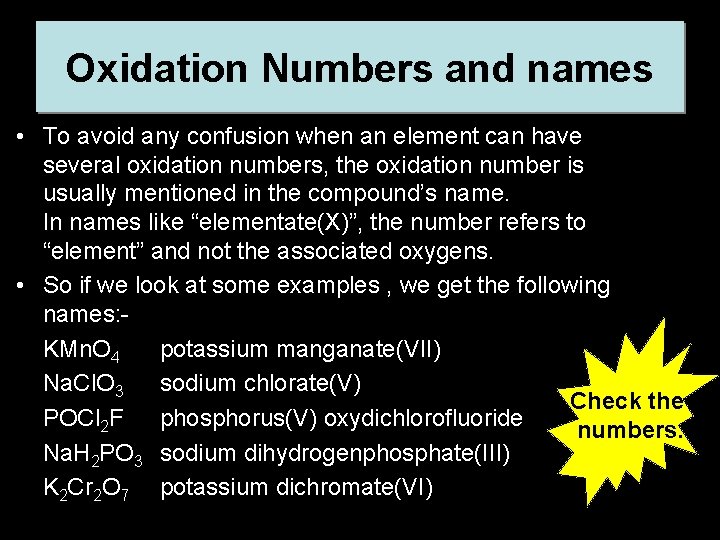
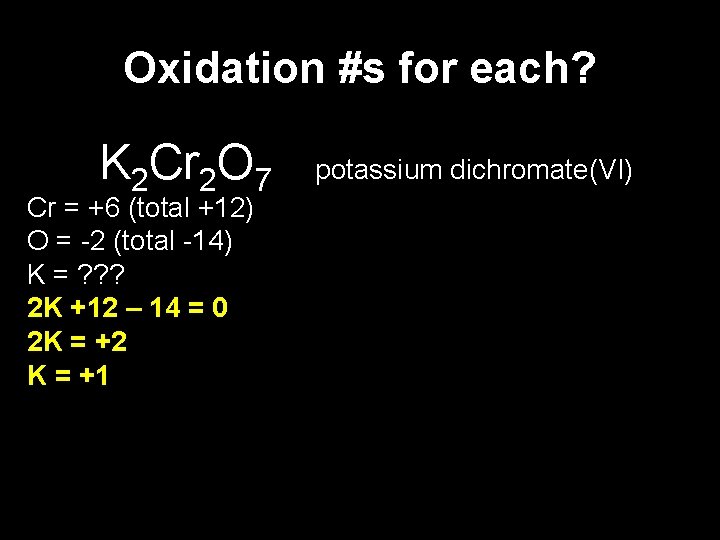
Oxidation Numbers and names • To avoid any confusion when an element can have several oxidation numbers, the oxidation number is usually mentioned in the compound’s name. In names like “elementate(X)”, the number refers to “element” and not the associated oxygens. • So if we look at some examples , we get the following names: KMn. O 4 potassium manganate(VII) Na. Cl. O 3 sodium chlorate(V) Check the POCl 2 F phosphorus(V) oxydichlorofluoride numbers. Na. H 2 PO 3 sodium dihydrogenphosphate(III) K 2 Cr 2 O 7 potassium dichromate(VI)

Oxidation #s for each? KMn. O 4 Na. Cl. O 3 POCl 2 F Na. H 2 PO 3 K 2 Cr 2 O 7 potassium manganate(VII) sodium chlorate(V) phosphorus(V) oxydichlorofluoride sodium dihydrogenphosphate(III) potassium dichromate(VI)

Oxidation #s for each? KMn. O 4 potassium manganate(VII) Mn = +7 O -2 total -8 K = ? ? ? K + 7 -8 = 0 K = +1

Oxidation #s for each? Na. Cl. O 3 sodium chlorate(V) Cl = +5 O = -2 (total -6) Na is Na + 5 – 6 = 0 Na = +1 (which it always is anyways)

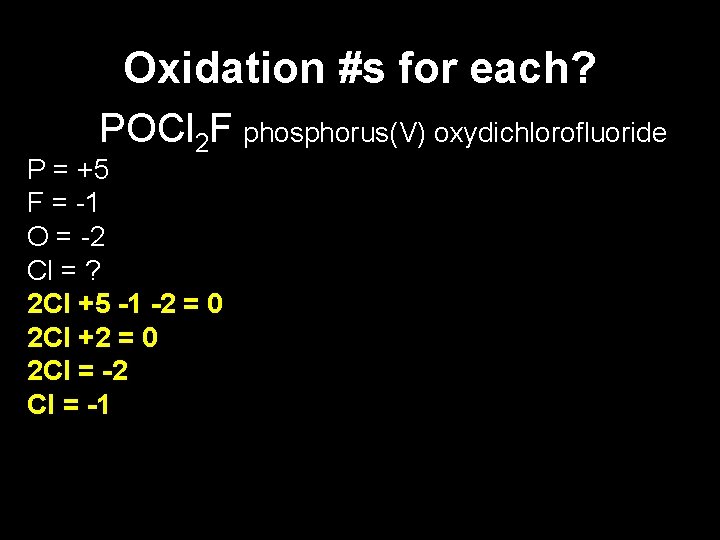
Oxidation #s for each? POCl 2 F phosphorus(V) oxydichlorofluoride P = +5 F = -1 O = -2 Cl = ? 2 Cl +5 -1 -2 = 0 2 Cl +2 = 0 2 Cl = -2 Cl = -1

Oxidation #s for each? Na. H 2 PO 3 P = +3 O = -2 (total -6) H = +1 (total +2) Na = ? ? ? Na +3 -6 +2 = 0 Na = +1 sodium dihydrogenphosphate(III)

Oxidation #s for each? K 2 Cr 2 O 7 Cr = +6 (total +12) O = -2 (total -14) K = ? ? ? 2 K +12 – 14 = 0 2 K = +2 K = +1 potassium dichromate(VI)

Well done!

Try Question #1 on homeworksheet