Redox Reactions Half Reaction One of the two






- Slides: 12

Redox Reactions

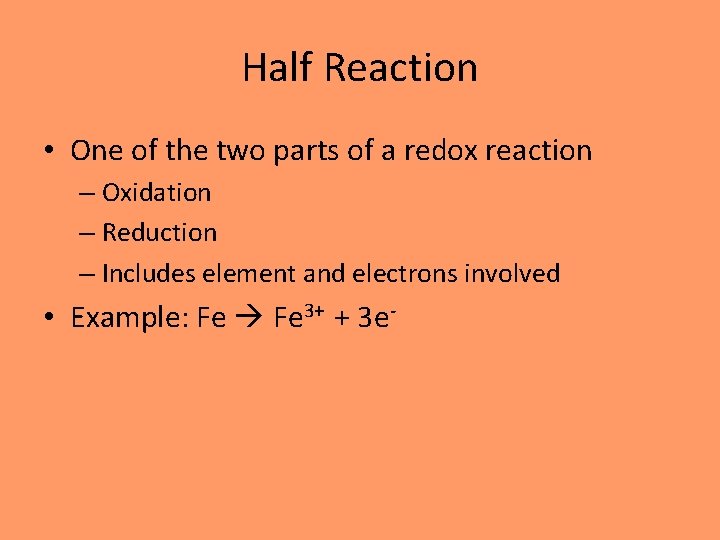
Half Reaction • One of the two parts of a redox reaction – Oxidation – Reduction – Includes element and electrons involved • Example: Fe 3+ + 3 e-

Example Write the half reaction for when the element chlorine is converted into the chloride ion.

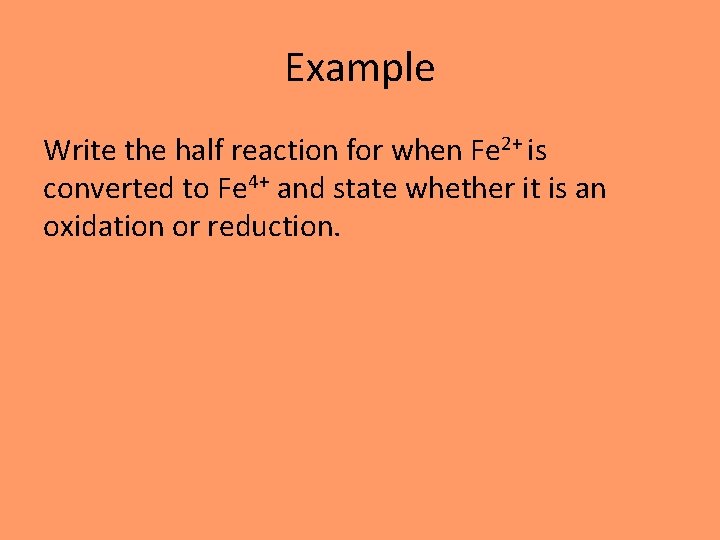
Example Write the half reaction for when Fe 2+ is converted to Fe 4+ and state whether it is an oxidation or reduction.

Redox Reactions • Oxidation and Reduction Reactions • Reaction in which electrons are transferred between atoms • Remember: • Oxidation is loss of electrons • Reduction is gain of electrons

• In a chemical reaction, oxidation cannot happen without reduction • Pneumonic: – LEO the lion says GER. • Loss electrons is oxidation, gain of electrons is reduction

Oxidation and Reduction in Redox Reactions • Assign oxidation numbers to each element in all compounds and elements • Values that change tell you what substance is oxidized and what substance is reduced

Example 2 K + Br 2 2 KBr • What is the charge of each element in the reaction? • What substance is reduced? • What substance is oxidized?

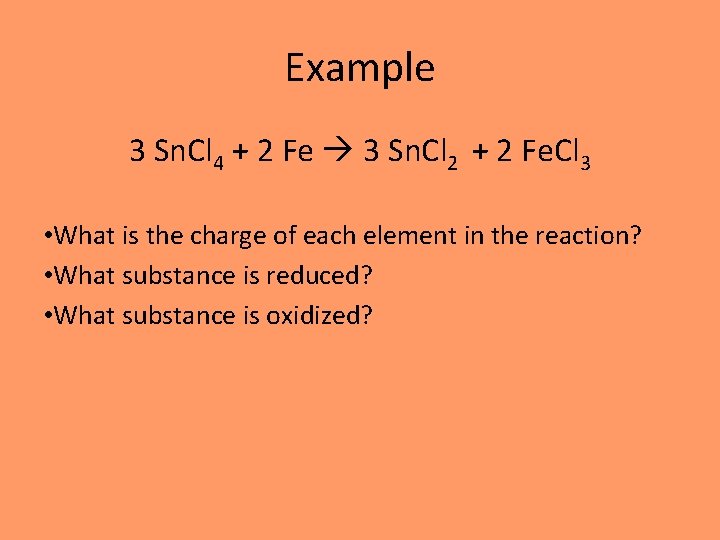
Example 3 Sn. Cl 4 + 2 Fe 3 Sn. Cl 2 + 2 Fe. Cl 3 • What is the charge of each element in the reaction? • What substance is reduced? • What substance is oxidized?

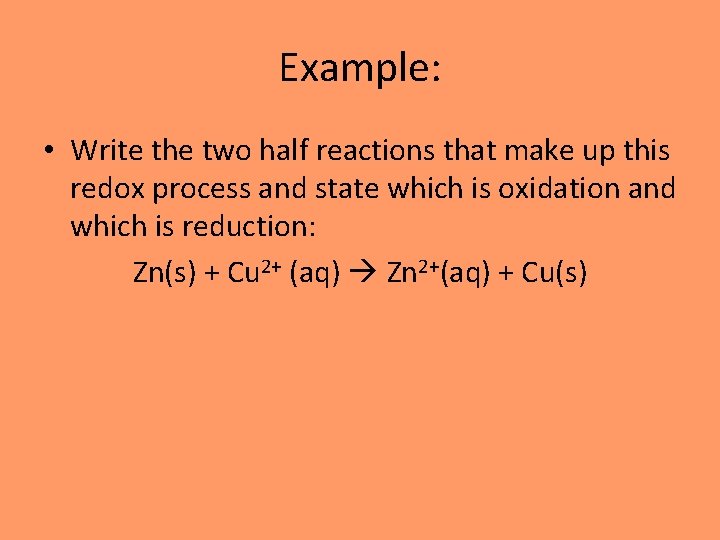
Example: • Write the two half reactions that make up this redox process and state which is oxidation and which is reduction: Zn(s) + Cu 2+ (aq) Zn 2+(aq) + Cu(s)

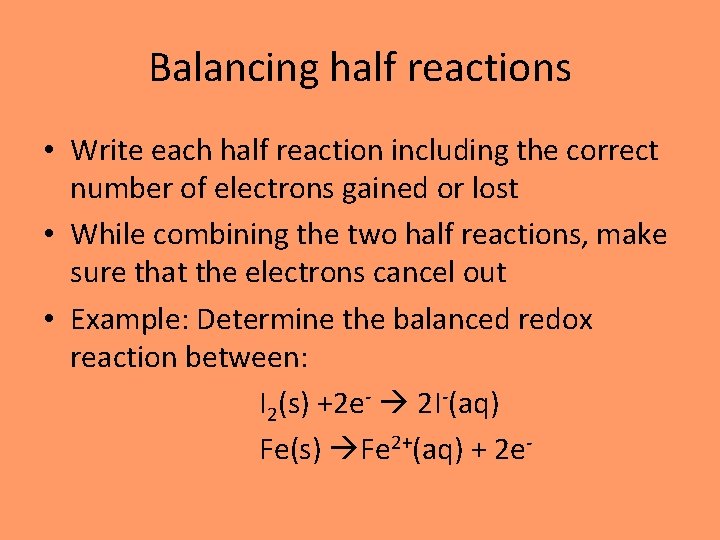
Balancing half reactions • Write each half reaction including the correct number of electrons gained or lost • While combining the two half reactions, make sure that the electrons cancel out • Example: Determine the balanced redox reaction between: I 2(s) +2 e- 2 I-(aq) Fe(s) Fe 2+(aq) + 2 e-

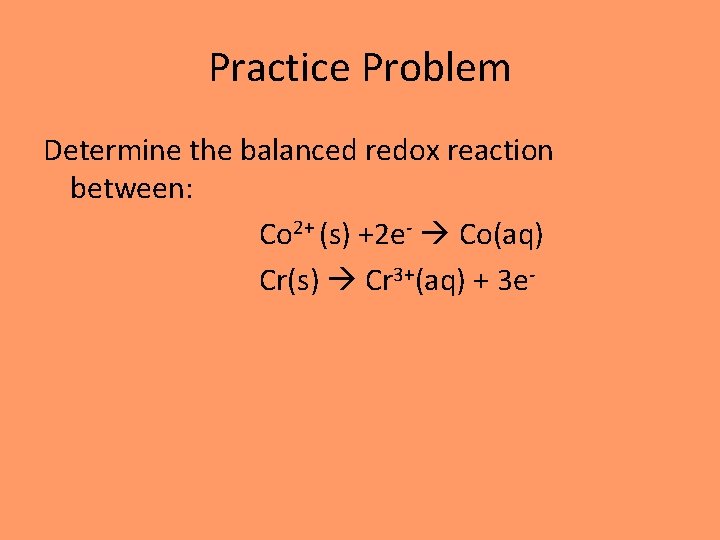
Practice Problem Determine the balanced redox reaction between: Co 2+ (s) +2 e- Co(aq) Cr(s) Cr 3+(aq) + 3 e-