Redox Reactions Examples 1 Synthesis The following is

- Slides: 7

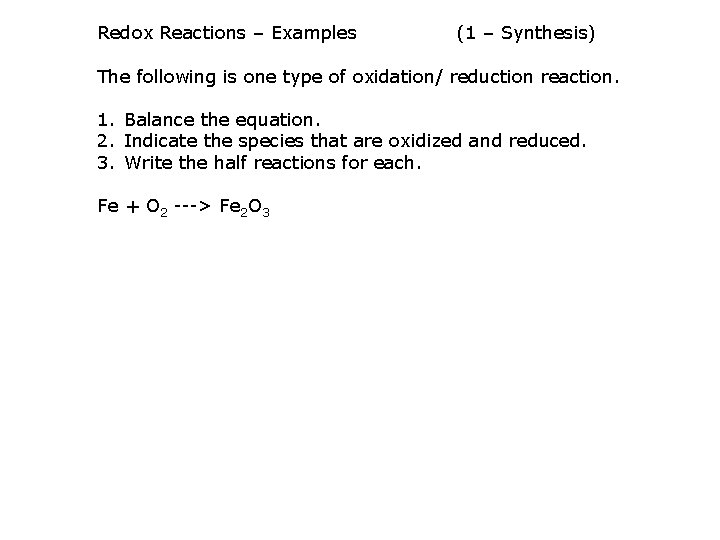
Redox Reactions – Examples (1 – Synthesis) The following is one type of oxidation/ reduction reaction. 1. Balance the equation. 2. Indicate the species that are oxidized and reduced. 3. Write the half reactions for each. Fe + O 2 ---> Fe 2 O 3

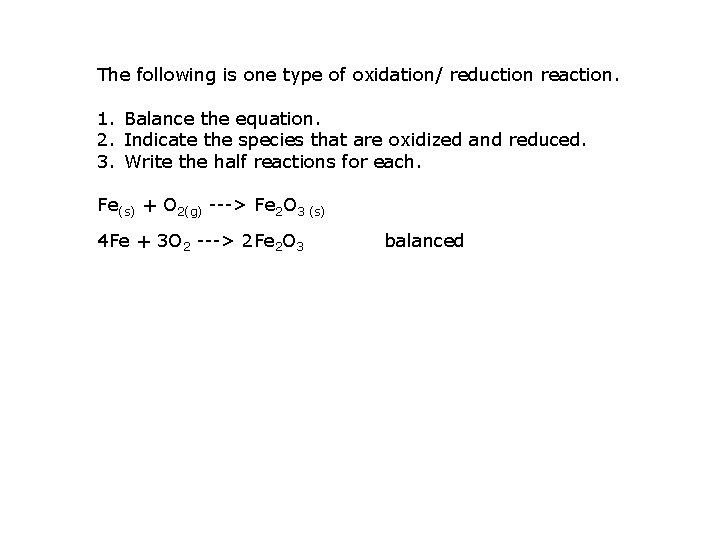
The following is one type of oxidation/ reduction reaction. 1. Balance the equation. 2. Indicate the species that are oxidized and reduced. 3. Write the half reactions for each. Fe(s) + O 2(g) ---> Fe 2 O 3 (s) 4 Fe + 3 O 2 ---> 2 Fe 2 O 3 balanced

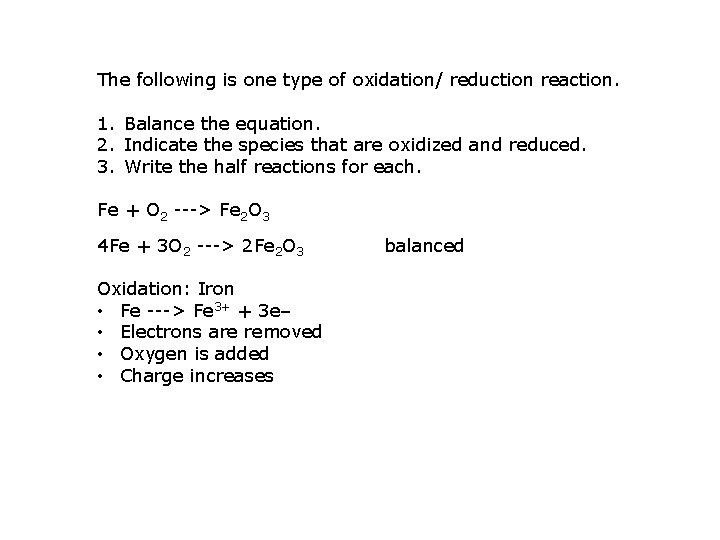
The following is one type of oxidation/ reduction reaction. 1. Balance the equation. 2. Indicate the species that are oxidized and reduced. 3. Write the half reactions for each. Fe + O 2 ---> Fe 2 O 3 4 Fe + 3 O 2 ---> 2 Fe 2 O 3 Oxidation: Iron • Fe ---> Fe 3+ + 3 e‒ • Electrons are removed • Oxygen is added • Charge increases balanced

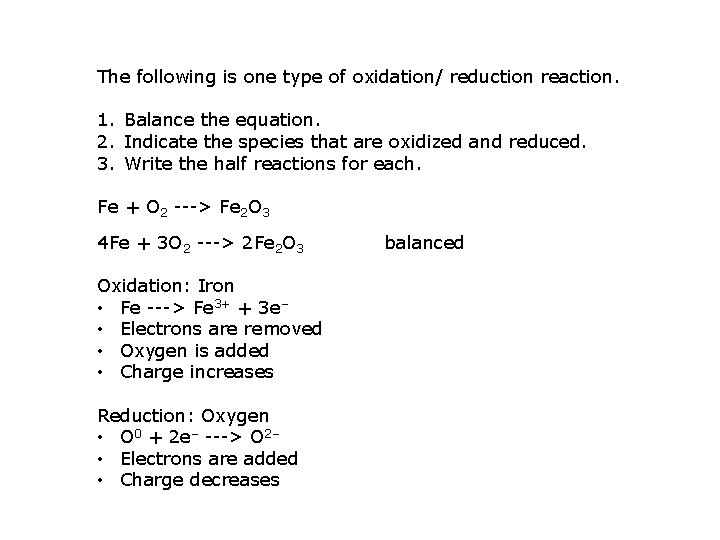
The following is one type of oxidation/ reduction reaction. 1. Balance the equation. 2. Indicate the species that are oxidized and reduced. 3. Write the half reactions for each. Fe + O 2 ---> Fe 2 O 3 4 Fe + 3 O 2 ---> 2 Fe 2 O 3 Oxidation: Iron • Fe ---> Fe 3+ + 3 e‒ • Electrons are removed • Oxygen is added • Charge increases Reduction: Oxygen • O 0 + 2 e‒ ---> O 2‒ • Electrons are added • Charge decreases balanced

Half Reactions: Fe 0 ---> Fe 3+ + 3 e‒ O 0 + 2 e‒ ---> O 2‒ Multiply coefficients through so that electrons cancel. 2 Fe 0 ---> 2 Fe 3+ + 6 e‒ 3 O 0 + 6 e‒ ---> 3 O 2‒ After electrons are cancelled: 2 Fe 0 ---> 2 Fe 3+ 3 O 0 ---> 3 O 2‒ Change elemental oxygen to diatomic, elemental. 2 Fe 0 ---> 2 Fe 3+ 1½ O 20 ---> 3 O 2‒ Multiply coefficients through so that they are whole numbers 4 Fe 0 ---> 4 Fe 3+ 3 O 20 ---> 6 O 2‒ Combine. 4 Fe + 3 O 2 ---> 4 Fe 3+ 6 O 2‒ Combine ions to make a compound, adjust coefficient. 4 Fe + 3 O 2 ---> 2 Fe 2 O 3

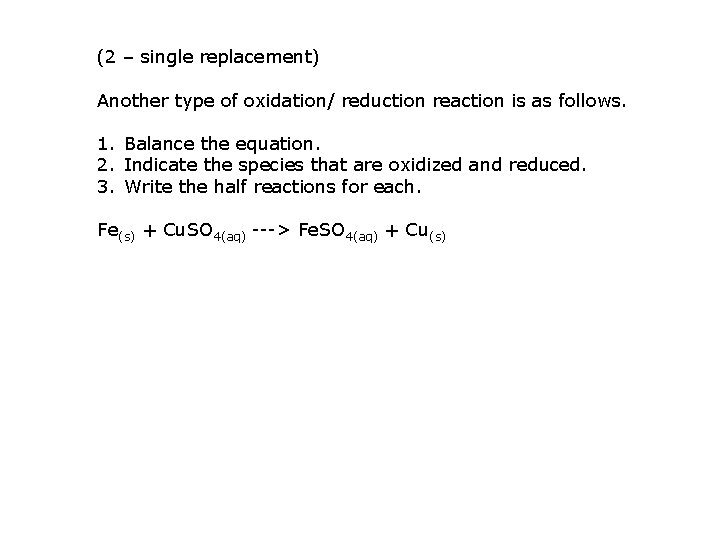
(2 – single replacement) Another type of oxidation/ reduction reaction is as follows. 1. Balance the equation. 2. Indicate the species that are oxidized and reduced. 3. Write the half reactions for each. Fe(s) + Cu. SO 4(aq) ---> Fe. SO 4(aq) + Cu(s)

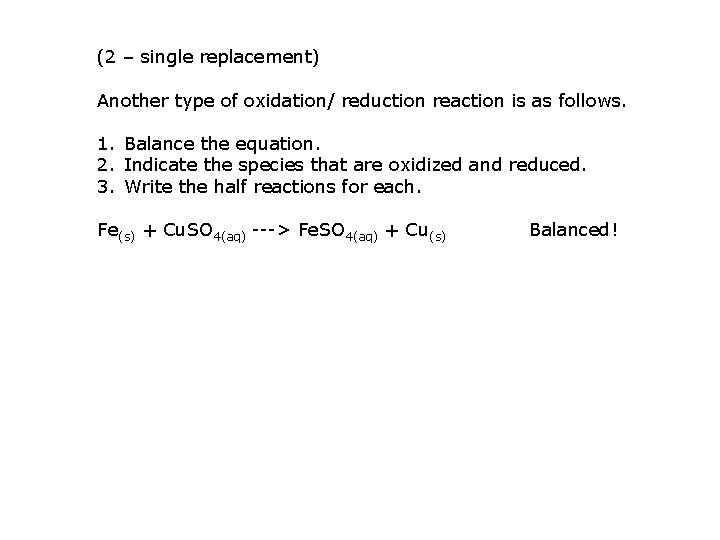
(2 – single replacement) Another type of oxidation/ reduction reaction is as follows. 1. Balance the equation. 2. Indicate the species that are oxidized and reduced. 3. Write the half reactions for each. Fe(s) + Cu. SO 4(aq) ---> Fe. SO 4(aq) + Cu(s) Balanced!