Redox Reactions EQ How does the transfer of














- Slides: 16

Redox Reactions EQ: How does the transfer of electrons drive redox reactions?

Review If an atom loses one electron, what will it’s charge/oxidation number be? What if it loses 3? What if it gains 2 electrons?

Redox Reactions Reduction-Oxidation Reactions Oxidation #’s change from reactants to products e- are being transferred

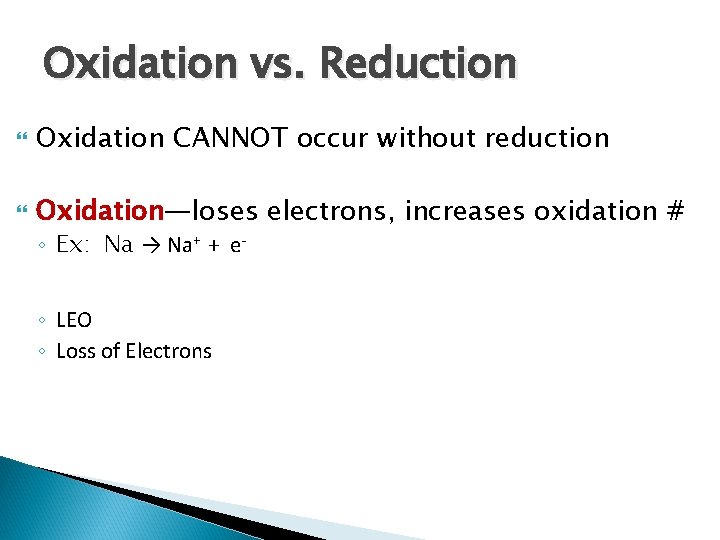
Oxidation vs. Reduction Oxidation CANNOT occur without reduction Oxidation—loses electrons, increases oxidation # ◦ Ex: Na → Na+ + e◦ LEO ◦ Loss of Electrons

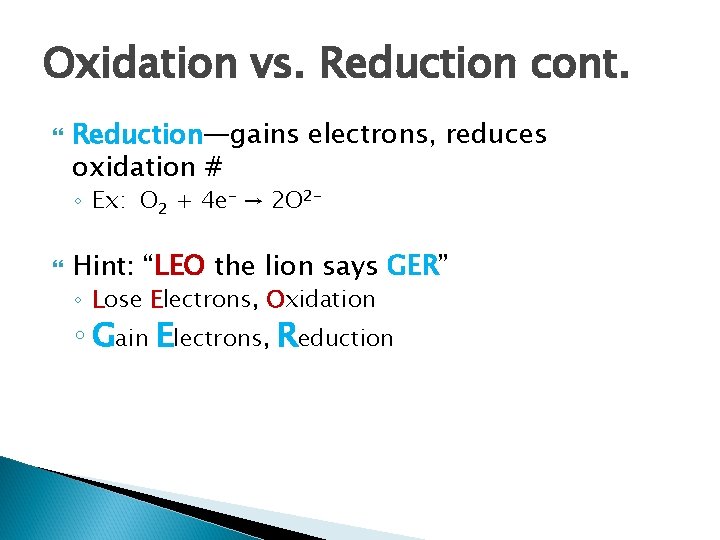
Oxidation vs. Reduction cont. Reduction—gains electrons, reduces oxidation # ◦ Ex: O 2 + 4 e- → 2 O 2 - Hint: “LEO the lion says GER” ◦ Lose Electrons, Oxidation ◦ Gain Electrons, Reduction

Assigning Oxidation #’s Pure elements = 0 ◦ Ex: H 2 is 0, as is Al Hydrogen is always +1 Oxygen is always -2, except in H 2 O 2 Group 1 is always +1, Group 2 always +2 Group 17 is always -1 Overall sum of ox. #’s: ◦ In a compound = 0 ◦ In a polyatomic ion = ion charge

Practice: NO P 4 O 8 (SO 4)-2

Classifying Species Oxidized species—substance being oxidized, losing electrons Reduced species—substance being reduced, gaining electrons Spectator ion—substance does NOT change Oxidizing agent—substance doing the oxidizing, accepting the electrons Reducing agent—substance doing the reducing, giving up the electrons

Redox Identification 1. 2. 3. 4. Assign oxidation #’s Identify the oxidized & reduced species Identify the oxidizing & reducing agents Write balanced half reactions

Practice: 2 Al + 3 Br 2 2 Al. Br 3 ◦ ◦ Oxidized species: Reduced species: Oxidizing agent: Reducing agent: 2 Mg + O 2 2 Mg. O ◦ ◦ Oxidized species: Reduced species: Oxidizing agent: Reducing agent:

Writing Half-Reactions 2 half-reactions per redox ◦ One represents oxidation ◦ One represents reduction Explicitly show exchange of e. Must be balanced!!

example Example: Al + Br 2 Al. Br 3 Balance the reaction, then determine which is oxidized, which is reduced?

Oxidation Half-Reactions e- added to product side Example: 2 Al + 3 Br 2 2 Al. Br 3 -Aluminum is being oxidized: 2 Al 3+ + 6 e-

Reduction Half-Reactions e- added to reactant side Example: 2 Al + 3 Br 2 2 Al. Br 3 -Bromine is being reduced: 6 Br + 6 e- 6 Br-

Putting it All Together 2 Al + 3 Br 2 2 Al 3+ + 6 e 6 Br + 6 e- 6 Br- 2 Al. Br 3

More Practice: Write half reactions for the following. Be sure to identify them as oxidation and reduction. S + O 2 SO 2 N 2 + 3 Ba Ba 3 N 2