Redox Reactions Chapter 20 Electron Transfer Redox Rxn








- Slides: 18

Redox Reactions Chapter 20

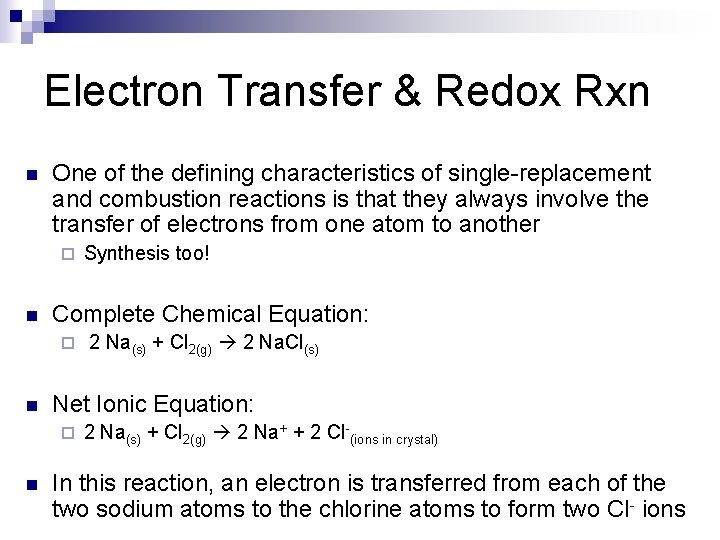
Electron Transfer & Redox Rxn n One of the defining characteristics of single-replacement and combustion reactions is that they always involve the transfer of electrons from one atom to another ¨ n Complete Chemical Equation: ¨ n 2 Na(s) + Cl 2(g) 2 Na. Cl(s) Net Ionic Equation: ¨ n Synthesis too! 2 Na(s) + Cl 2(g) 2 Na+ + 2 Cl-(ions in crystal) In this reaction, an electron is transferred from each of the two sodium atoms to the chlorine atoms to form two Cl- ions

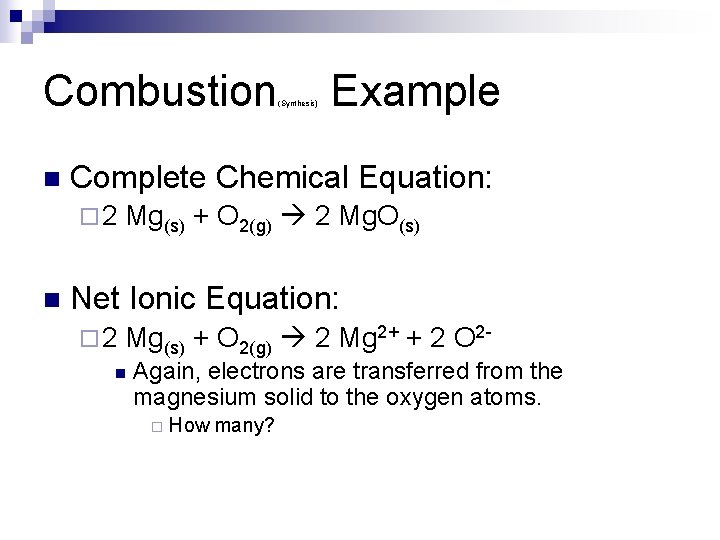
Combustion n Example Complete Chemical Equation: ¨ 2 n (Synthesis) Mg(s) + O 2(g) 2 Mg. O(s) Net Ionic Equation: ¨ 2 Mg(s) + O 2(g) 2 Mg 2+ + 2 O 2 - n Again, electrons are transferred from the magnesium solid to the oxygen atoms. ¨ How many?

Redox Reactions n A reaction in which electrons are transferred from one atom to another is called an oxidation-reduction reaction ¨ n We use Redox for simplicity How do oxidation and reduction differ? ¨ Oxidation is referred to as the loss of electrons from atoms of a substance n ¨ Na Na+ + e- Reduction is defined as the gain of electrons by atoms of a substance n Cl 2 + 2 e- 2 Cl-

Redox Reactions n An easy way to remember the difference is the saying: ¨ LEO the lion says GER or LEO n Loss of Electrons is Oxidation n Gain of Electrons is Reduction n GER Remember - The oxidation number of an atom is the charge made by ions forming ¨ Group 1 is +1, Group 2 is +2 ¨ Nobel Gases are 0, Group 17 is -1, etc.

Oxidizing and Reducing Agents n In Redox Reactions, one substance will act as the oxidizing agent. ¨ Thus the substance that oxidizes another substance by accepting its electrons is called an oxidizing agent ¨ The reducing agent is the substance that reduces another substance by losing electrons n A disproportionation is a substance that acts as both a reducing and oxidizing agent. oxidized n 2 K(s) + Br 2(g) 2 KBr(s) reduced Potassium was oxidized so it is the reducing agent Bromine was reduced so it is the oxidizing agent

Redox and Electronegativity n Recall: ¨ Electronegativity is an atom in a chemical compound that wants to have electrons The trend on the PT increases to the upper right, not Nobel Gases n These will create partial charges n

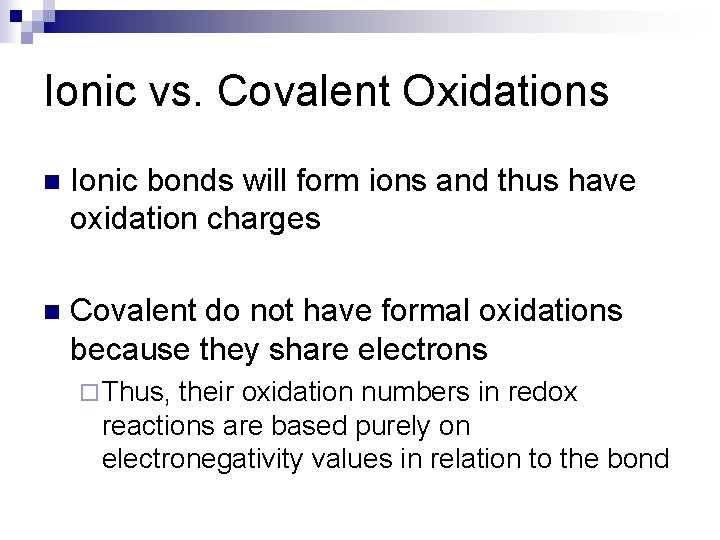
Ionic vs. Covalent Oxidations n Ionic bonds will form ions and thus have oxidation charges n Covalent do not have formal oxidations because they share electrons ¨ Thus, their oxidation numbers in redox reactions are based purely on electronegativity values in relation to the bond

Determining an Oxidation Numbers n Rules for Determining Oxidation Numbers 1. The oxidation number of an uncombined atom is zero. This is also true for polyatomic molecules such as O 2, Cl 2, S 8, etc. The oxidation number of a monatomic ion is equal to the charge on the ion Na 1+ is +1, Cl 1 - is -1 The oxidation number of the more electronegative atom in a molecule or a complex ion is the same as the charge it would have if it were an ion The most electronegative element, fluorine, always has an oxidation number of 1 when it is bonded to another element The oxidation number of oxygen in compounds is always -2, except in peroxides (H 2 O 2) and when bonded to the only element more electronegative than itself, F (it will be +2 in this case) The oxidation number of hydrogen in most cases is +1, unless its bonded to a less electronegative atom such as metals in group 1, 2 and 13 The metals of groups 1 and 2 and Aluminum in 13 are still always +1, +2 and +3, respectively The sum of the oxidation numbers in a neutral compound must be zero The sum of oxidation numbers of the atoms in a polyatomic ion is equal to the charge on the ion 2. 3. 4. 5. 6. 7. 8. 9.

Balancing Redox Reactions n Simple redox reactions require the inspection technique ¨ The skill we have been using this year, which is done by just counting up the atoms and balancing them on both sides n More complex redox reactions will require a more systematic (mathematical) approach ¨ Oxidation Number Method ¨ Equation-Balance Method

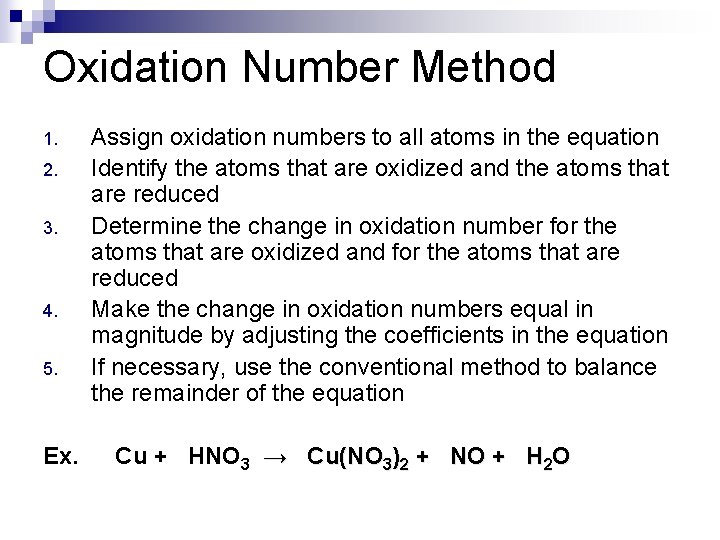
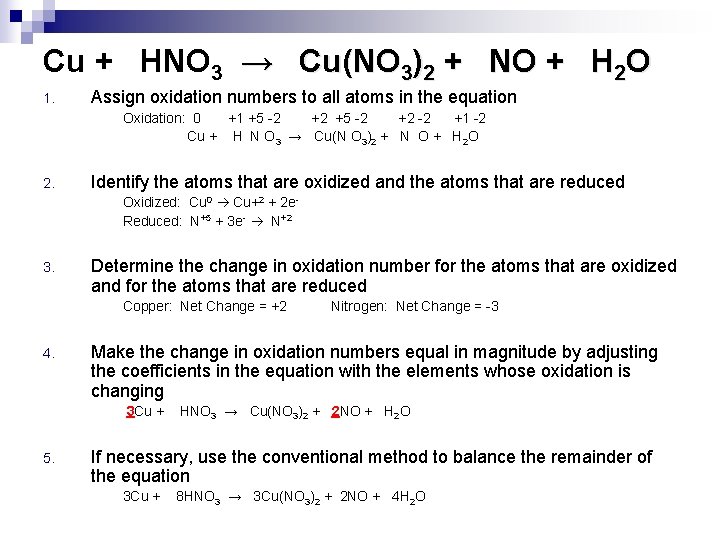
Oxidation Number Method 1. 2. 3. 4. 5. Ex. Assign oxidation numbers to all atoms in the equation Identify the atoms that are oxidized and the atoms that are reduced Determine the change in oxidation number for the atoms that are oxidized and for the atoms that are reduced Make the change in oxidation numbers equal in magnitude by adjusting the coefficients in the equation If necessary, use the conventional method to balance the remainder of the equation Cu + HNO 3 → Cu(NO 3)2 + NO + H 2 O

Cu + HNO 3 → Cu(NO 3)2 + NO + H 2 O 1. Assign oxidation numbers to all atoms in the equation Oxidation: 0 +1 +5 -2 +2 -2 +1 -2 Cu + H N O 3 → Cu(N O 3)2 + N O + H 2 O 2. Identify the atoms that are oxidized and the atoms that are reduced Oxidized: Cu 0 Cu+2 + 2 e. Reduced: N+5 + 3 e- N+2 3. Determine the change in oxidation number for the atoms that are oxidized and for the atoms that are reduced Copper: Net Change = +2 4. Make the change in oxidation numbers equal in magnitude by adjusting the coefficients in the equation with the elements whose oxidation is changing 3 Cu + 5. Nitrogen: Net Change = -3 HNO 3 → Cu(NO 3)2 + 2 NO + H 2 O If necessary, use the conventional method to balance the remainder of the equation 3 Cu + 8 HNO 3 → 3 Cu(NO 3)2 + 2 NO + 4 H 2 O

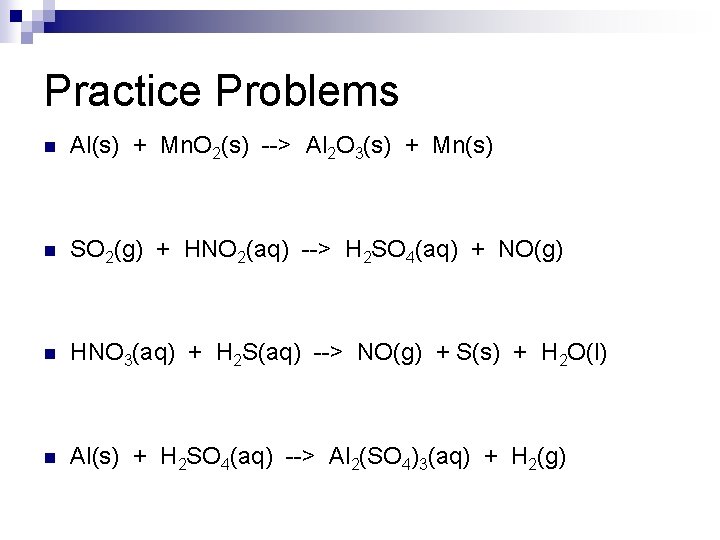
Practice Problems n Al(s) + Mn. O 2(s) --> Al 2 O 3(s) + Mn(s) n SO 2(g) + HNO 2(aq) --> H 2 SO 4(aq) + NO(g) n HNO 3(aq) + H 2 S(aq) --> NO(g) + S(s) + H 2 O(l) n Al(s) + H 2 SO 4(aq) --> Al 2(SO 4)3(aq) + H 2(g)

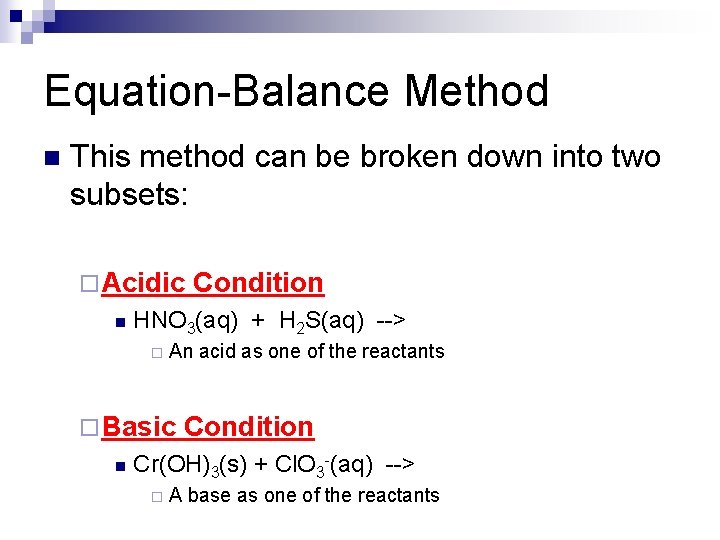
Equation-Balance Method n This method can be broken down into two subsets: ¨ Acidic n HNO 3(aq) + H 2 S(aq) --> ¨ An acid as one of the reactants ¨ Basic n Condition Cr(OH)3(s) + Cl. O 3 -(aq) --> ¨ A base as one of the reactants

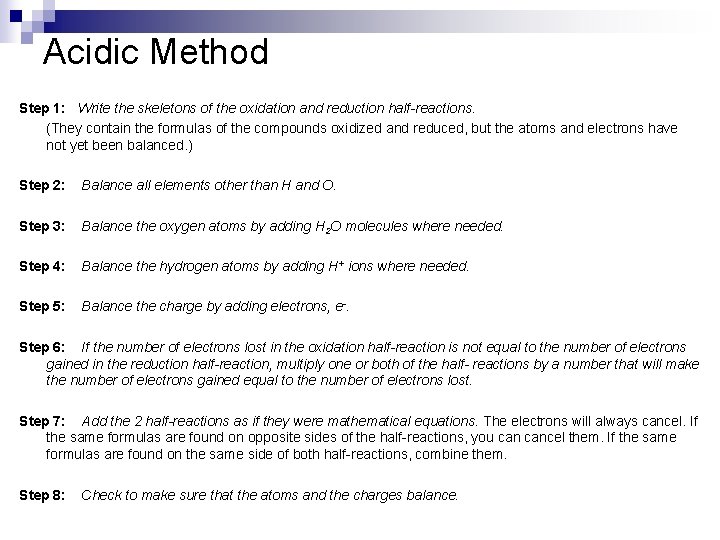
Acidic Method Step 1: Write the skeletons of the oxidation and reduction half-reactions. (They contain the formulas of the compounds oxidized and reduced, but the atoms and electrons have not yet been balanced. ) Step 2: Balance all elements other than H and O. Step 3: Balance the oxygen atoms by adding H 2 O molecules where needed. Step 4: Balance the hydrogen atoms by adding H+ ions where needed. Step 5: Balance the charge by adding electrons, e-. Step 6: If the number of electrons lost in the oxidation half-reaction is not equal to the number of electrons gained in the reduction half-reaction, multiply one or both of the half- reactions by a number that will make the number of electrons gained equal to the number of electrons lost. Step 7: Add the 2 half-reactions as if they were mathematical equations. The electrons will always cancel. If the same formulas are found on opposite sides of the half-reactions, you cancel them. If the same formulas are found on the same side of both half-reactions, combine them. Step 8: Check to make sure that the atoms and the charges balance.

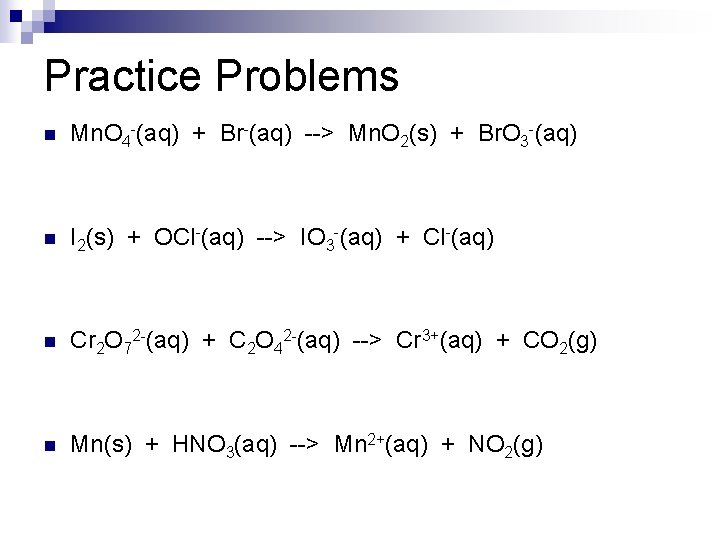
Practice Problems n Mn. O 4 -(aq) + Br-(aq) --> Mn. O 2(s) + Br. O 3 -(aq) n I 2(s) + OCl-(aq) --> IO 3 -(aq) + Cl-(aq) n Cr 2 O 72 -(aq) + C 2 O 42 -(aq) --> Cr 3+(aq) + CO 2(g) n Mn(s) + HNO 3(aq) --> Mn 2+(aq) + NO 2(g)

Basic Method Steps 1 -7: Begin by balancing the equation as if it were in acid solution. If you have H+ ions in your equation at the end of these steps, proceed to Step #8. Otherwise, skip to Step #11. Step 8: Add enough OH- ions to each side to cancel the H+ ions. (Be sure to add the OH- ions to both sides to keep the charge and atoms balanced. ) Step 9: Combine the H+ ions and OH- ions that are on the same side of the equation to form water. Step 10: Cancel or combine the H 2 O molecules. Step 11: Check to make sure that the atoms and the charge balance. If they do balance, you are done. If they do not balance, re-check your work in Steps 1 -10.

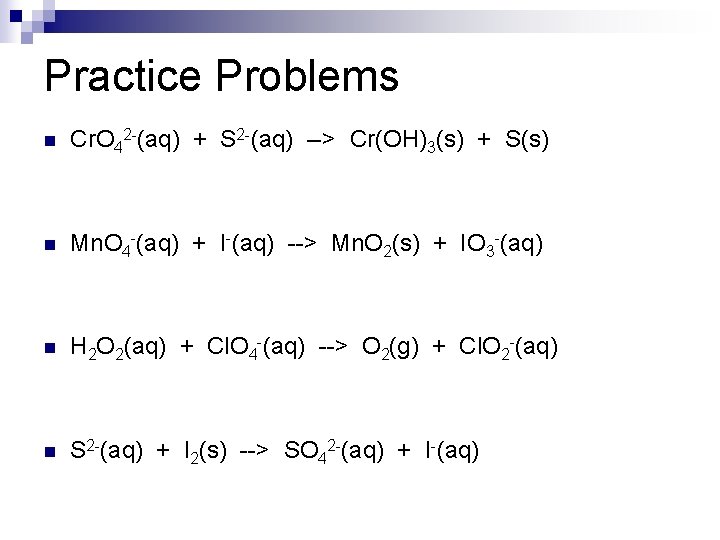
Practice Problems n Cr. O 42 -(aq) + S 2 -(aq) --> Cr(OH)3(s) + S(s) n Mn. O 4 -(aq) + I-(aq) --> Mn. O 2(s) + IO 3 -(aq) n H 2 O 2(aq) + Cl. O 4 -(aq) --> O 2(g) + Cl. O 2 -(aq) n S 2 -(aq) + I 2(s) --> SO 42 -(aq) + I-(aq)