Redox Reactions 13 1 a Electron Transfer Theory


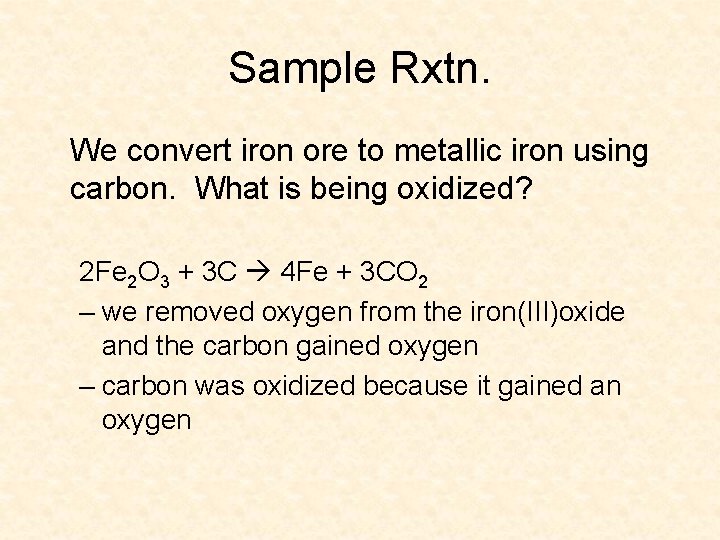


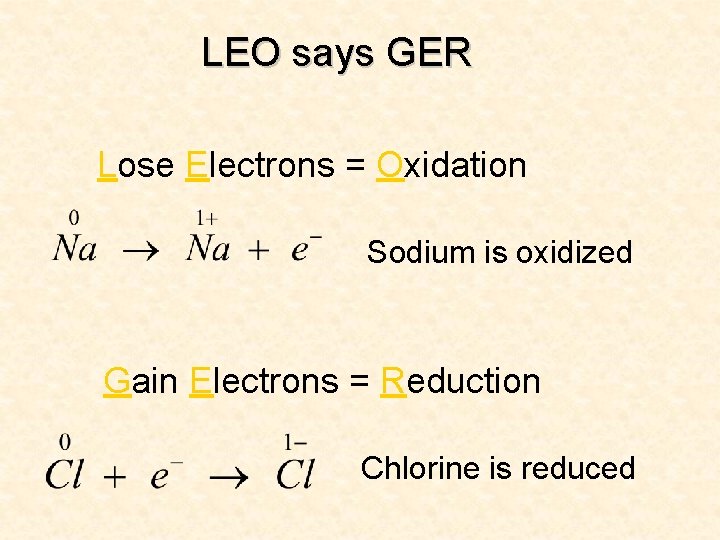



- Slides: 18

Redox Reactions 13. 1 a Electron Transfer Theory

Overview • oxidation and reduction rxtns always occur together (redox rxtn) – you can’t have one without the other • examples – extracting metal from an ore – electroplating of metals – salt on roads (corrosion) • salt accelerates the reaction between oxygen and metal • makes electron transfer easier

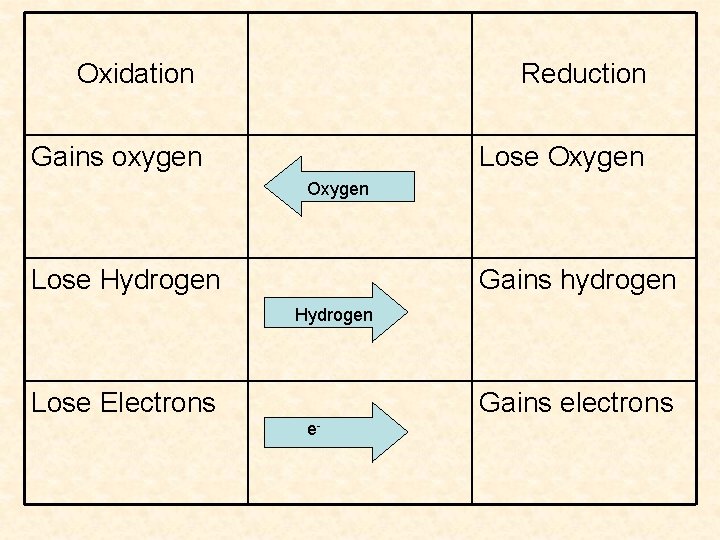
3 perspectives of oxidation/reduction • 1) oxygen – oxidation gains and reduction loses • 2) hydrogen – oxidation loses and reduction gains • 3) electrons (modern view) – oxidation loses and reduction gains – most fundamental explanation, what we will be working with the most
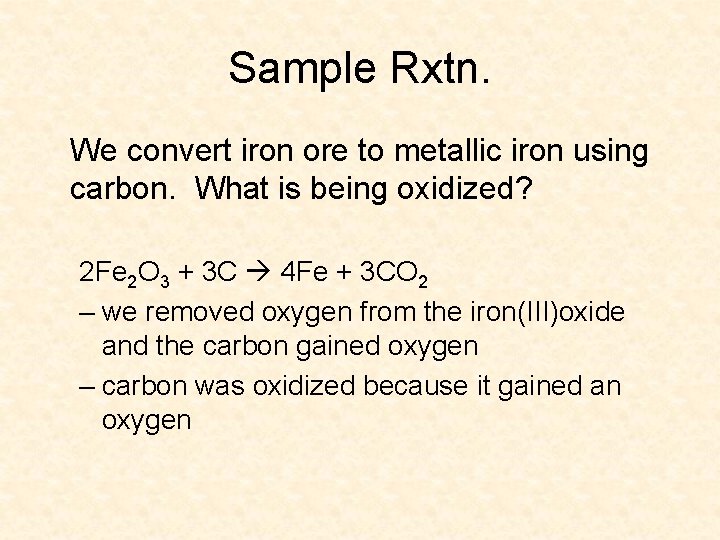
Sample Rxtn. We convert iron ore to metallic iron using carbon. What is being oxidized? 2 Fe 2 O 3 + 3 C 4 Fe + 3 CO 2 – we removed oxygen from the iron(III)oxide and the carbon gained oxygen – carbon was oxidized because it gained an oxygen

Sample Rxtns. con’t Fe 2 O 3 (s) + 3 CO(g) 2 Fe (s) + 3 CO 2 (g) Sn. O 2 (s) + C(s) Sn(s) + CO 2 (g) Cu. S(s) + H 2(g) Cu(s) + H 2 S(g)

Oxidation Reduction Gains oxygen Lose Oxygen Lose Hydrogen Gains hydrogen Hydrogen Lose Electrons Gains electrons e-

Redox with Electrons many reactions may not even involve oxygen redox currently refers to electrons being transferred between reactants Mg + S Mg 2+ + S 2 - (Mg. S) • the magnesium atom (which has zero charge) changes to a magnesium ion by losing 2 electrons, and is oxidized to Mg 2+ • the sulfur atom (which has no charge) is changed to a sulfide ion by gaining 2 electrons, and is reduced to S 2 • Mg is the reducing agent and S is the oxidizing agent • agents cause the other half of the rxtn. to occur

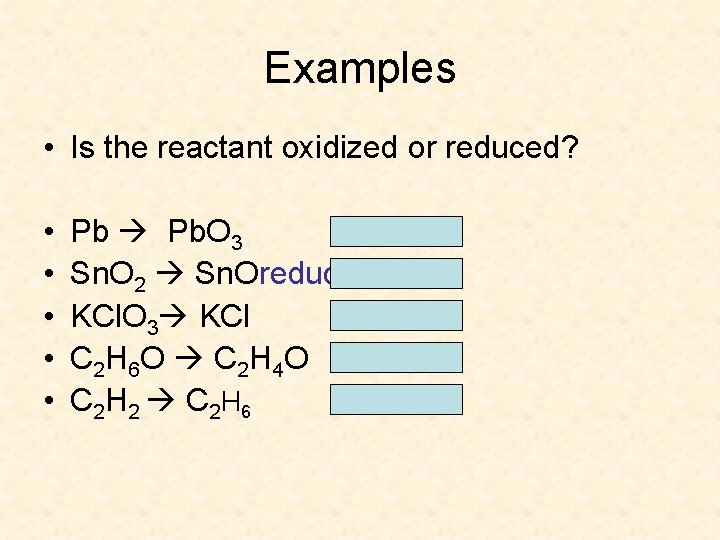
Examples • Is the reactant oxidized or reduced? • • • Pb. O 3 oxidized Sn. O 2 Sn. Oreduced KCl. O 3 KCl reduced C 2 H 6 O C 2 H 4 O oxidized C 2 H 2 C 2 H 6 reduced

Homework Textbook p. 559 #1 -4

Redox Reactions 13. 1 a 1 Electron Transfer Theory

Leo the Lion! • LEO the lion says GER – Loss of electrons is oxidation, gain of electrons is reduction
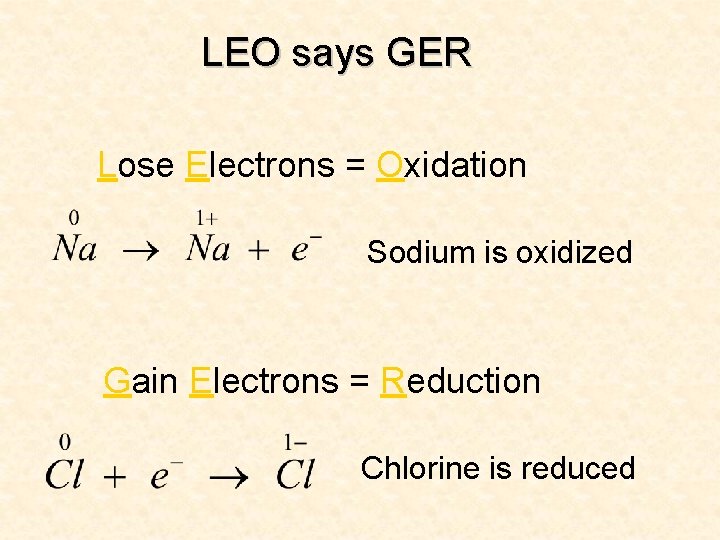
LEO says GER Lose Electrons = Oxidation Sodium is oxidized Gain Electrons = Reduction Chlorine is reduced

LEO says GER con’t - losing electrons is oxidation, and the substance that loses the electrons is called the reducing agent. - gaining electrons is reduction, and the substance that gains the electrons is called the oxidizing agent. Mg is the reducing agent Mg is oxidized: loses e-, becomes a Mg 2+ ion Mg(s) + S(s) S is the oxidizing agent Mg. S(s) S is reduced: gains e- = S 2 - ion

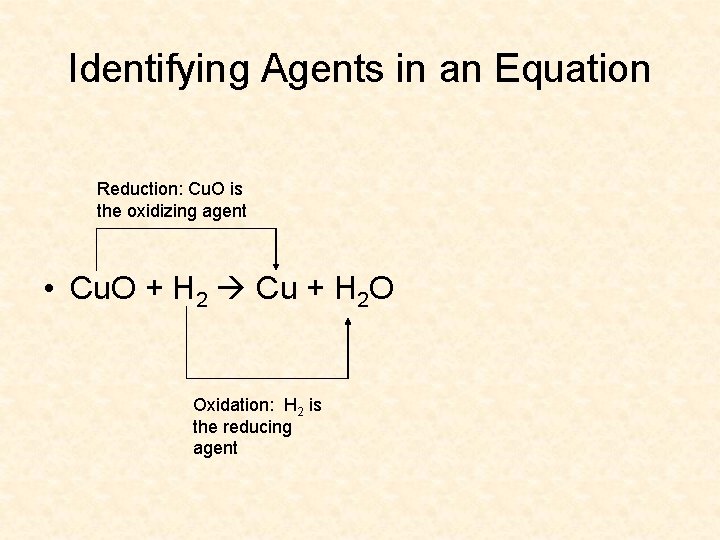
Oxidizing and Reducing Agents Cu. O + H 2 Cu + H 2 O • Cu goes from 2+ to 0 – Cu is reduced, therefore it is called an oxidizing agent because it causes some other substance to be oxidized • H goes from 0 to 1+ – H is oxidized, therefore it is called a reducing agent because it causes some other substance to be reduced

Identifying Agents in an Equation Reduction: Cu. O is the oxidizing agent • Cu. O + H 2 Cu + H 2 O Oxidation: H 2 is the reducing agent

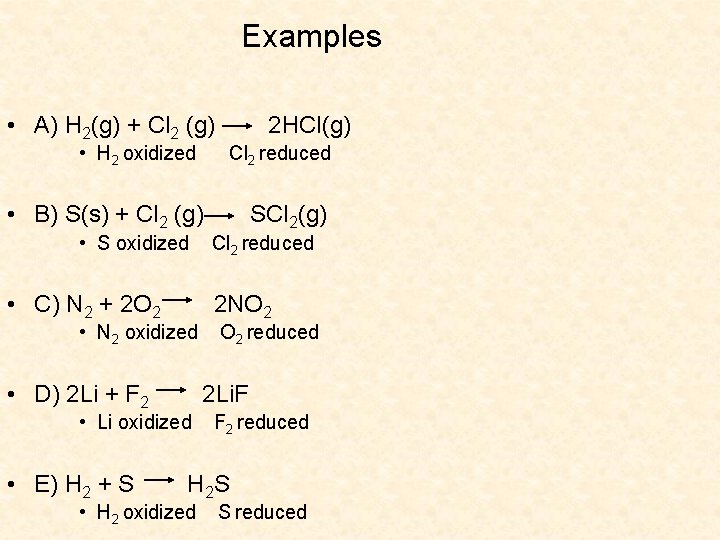
Examples • A) H 2(g) + Cl 2 (g) • H 2 oxidized • B) S(s) + Cl 2 (g) • S oxidized • C) N 2 + 2 O 2 • N 2 oxidized • D) 2 Li + F 2 Cl 2 reduced SCl 2(g) Cl 2 reduced 2 NO 2 reduced 2 Li. F • Li oxidized • E) H 2 + S 2 HCl(g) F 2 reduced H 2 S • H 2 oxidized S reduced

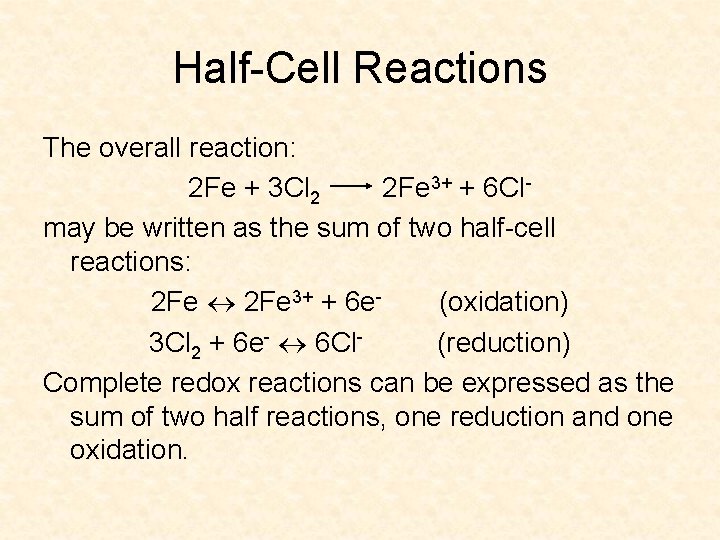
Half-Cell Reactions The overall reaction: 2 Fe + 3 Cl 2 2 Fe 3+ + 6 Clmay be written as the sum of two half-cell reactions: 2 Fe 3+ + 6 e(oxidation) 3 Cl 2 + 6 e- 6 Cl(reduction) Complete redox reactions can be expressed as the sum of two half reactions, one reduction and one oxidation.

Homework Textbook p. 564 #7 -11 LSM 13. 1 B, C summary