REDOX REACTION REDOX REACTION Chemical reactions which involves





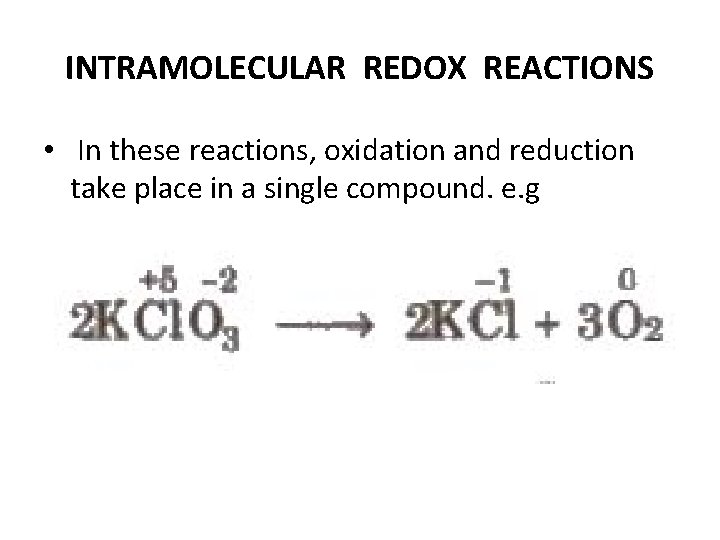



- Slides: 25

REDOX REACTION

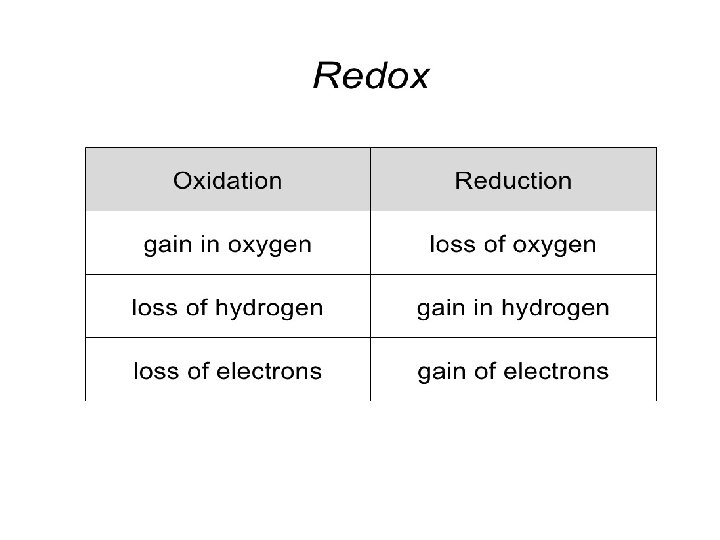
REDOX REACTION • Chemical reactions which involves both oxidation as well as reduction process simultaneously, are known as redox reactions (‘red’) from reduction and ‘ox’ from oxidation) • All these reactions are always accompanied by energy change in the form of heat, light or electricity


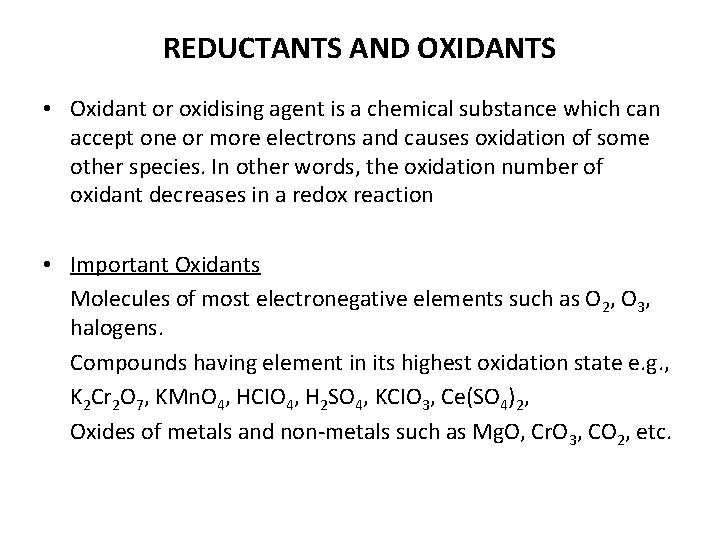
REDUCTANTS AND OXIDANTS • Oxidant or oxidising agent is a chemical substance which can accept one or more electrons and causes oxidation of some other species. In other words, the oxidation number of oxidant decreases in a redox reaction • Important Oxidants Molecules of most electronegative elements such as O 2, O 3, halogens. Compounds having element in its highest oxidation state e. g. , K 2 Cr 2 O 7, KMn. O 4, HCIO 4, H 2 SO 4, KCIO 3, Ce(SO 4)2, Oxides of metals and non-metals such as Mg. O, Cr. O 3, CO 2, etc.

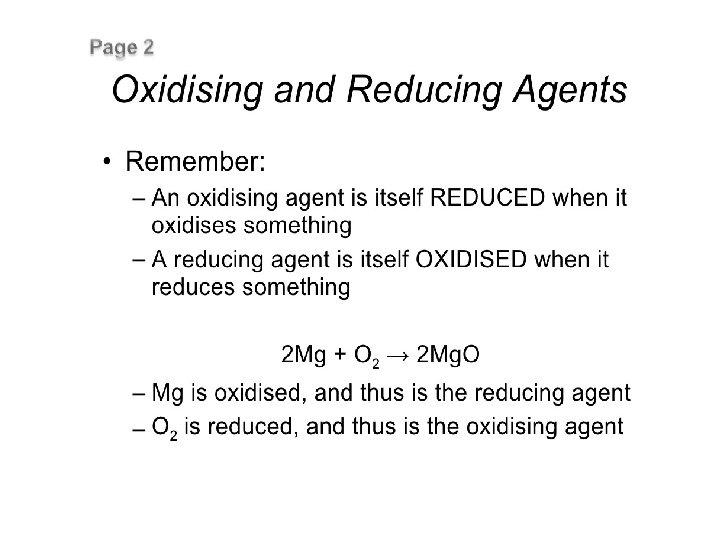
REDUCTANT OR REDUCING AGENT • Reductant or reducing agent is a chemical substance which can give one or more electrons and causes reduction of some other species. In other words, the oxidation number of reductant increases in a redox reaction. • Important Reductants All metals such as Na, AI, Zn, etc. , and some non – metals, e. g. , C, S. P, H 2, etc. Metallic hydrides like Na. H, Li. H. KH, Ca. H 2 etc.


OXIDATION NUMBER • The oxidation number is defined as the charge in which an atom appears to have when all other atoms are removed from it as ions. It may have + or – sign.

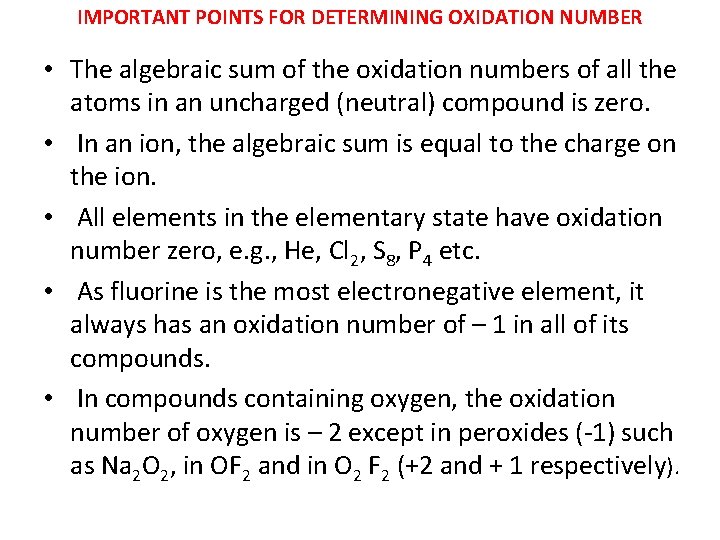
IMPORTANT POINTS FOR DETERMINING OXIDATION NUMBER • The algebraic sum of the oxidation numbers of all the atoms in an uncharged (neutral) compound is zero. • In an ion, the algebraic sum is equal to the charge on the ion. • All elements in the elementary state have oxidation number zero, e. g. , He, Cl 2, S 8, P 4 etc. • As fluorine is the most electronegative element, it always has an oxidation number of – 1 in all of its compounds. • In compounds containing oxygen, the oxidation number of oxygen is – 2 except in peroxides (-1) such as Na 2 O 2, in OF 2 and in O 2 F 2 (+2 and + 1 respectively).

• In all compounds. except ionic metallic hydrides, the oxidation number of hydrogen is +1. In metal hydrides like Na. H, Mg. H 2, Ca. H 2, Li. H, etc the oxidation number of hydrogen is -1. • Oxidation number for alkali metals is +1 and for alkaline earth metals is + 2. • If coordinate bond is directed from more electronegative to less electronegative atom then its contribution be zero for both the atoms. • For p-block elements [Except F and 0], the highest oxidation number is equal to their group number and lowest oxidation number is equal to the group number minus eight. • In transition elements the lowest oxidation number is equal to the number of ns electrons and highest oxidation number is equal to number of ‘ns’ and (n – l)d unpaired electrons.

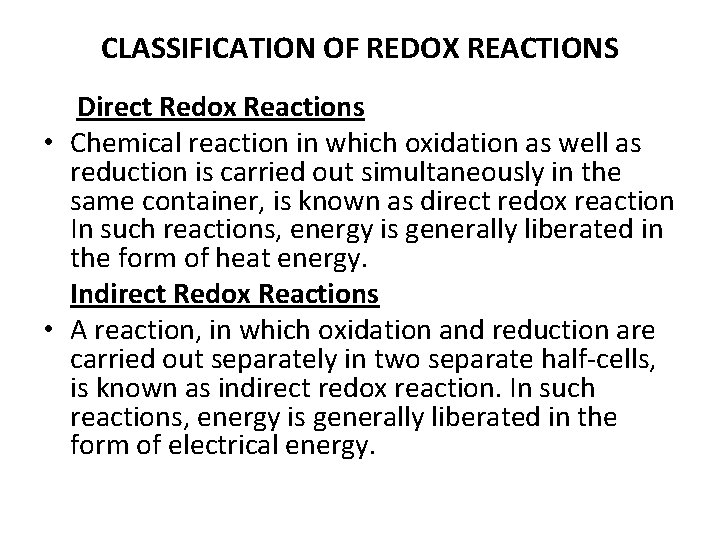
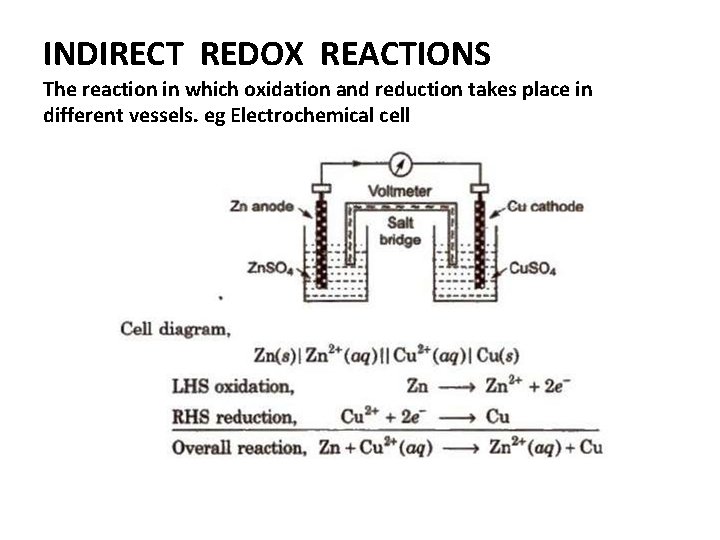
CLASSIFICATION OF REDOX REACTIONS Direct Redox Reactions • Chemical reaction in which oxidation as well as reduction is carried out simultaneously in the same container, is known as direct redox reaction In such reactions, energy is generally liberated in the form of heat energy. Indirect Redox Reactions • A reaction, in which oxidation and reduction are carried out separately in two separate half-cells, is known as indirect redox reaction. In such reactions, energy is generally liberated in the form of electrical energy.

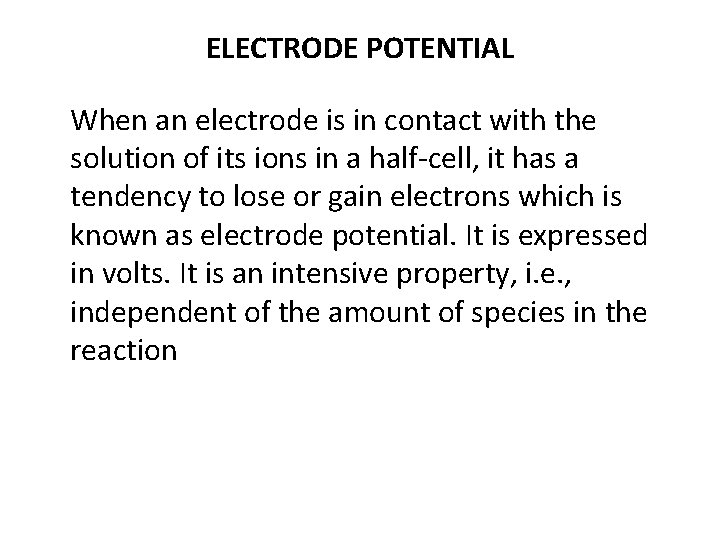
ELECTRODE POTENTIAL When an electrode is in contact with the solution of its ions in a half-cell, it has a tendency to lose or gain electrons which is known as electrode potential. It is expressed in volts. It is an intensive property, i. e. , independent of the amount of species in the reaction

• Oxidation potential The tendency to lose electrons is known as oxidation potential. Oxidation potential of a half-cell is inversely proportional to the concentration of ions in the solution. • Reduction potential The tendency to gain electrons is known as reduction potential. According to IUPAC convention, the reduction potential alone be called as the electrode potential unless it is specifically mentioned. E°red = – E°oxidalion

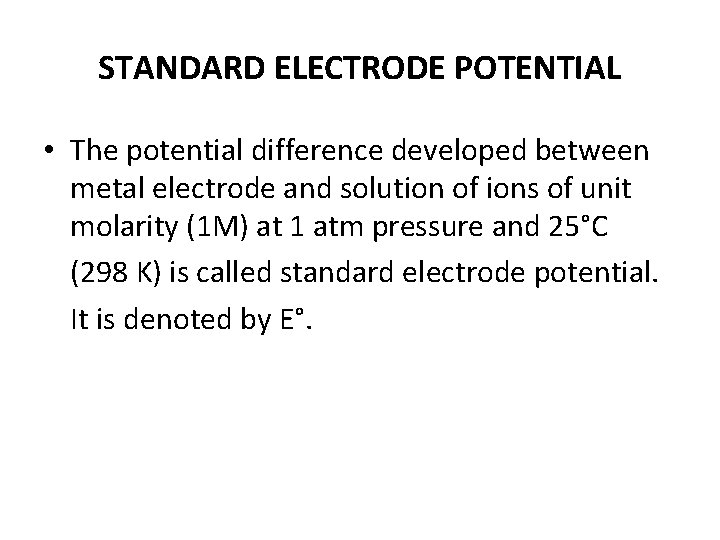
STANDARD ELECTRODE POTENTIAL • The potential difference developed between metal electrode and solution of ions of unit molarity (1 M) at 1 atm pressure and 25°C (298 K) is called standard electrode potential. It is denoted by E°.

INDIRECT REDOX REACTIONS The reaction in which oxidation and reduction takes place in different vessels. eg Electrochemical cell

Function of salt bridge • It completes the circuit and allows the flow of current. • It maintains the electrical neutrality on both sides. Salt-bridge generally contains • solution of strong electrolyte such as KNO 3, KCl etc. KCI is preferred because the transport numbers of K+ and Cl-are almost same.

REFERENCE ELECTRODE The electrode of known potential is called reference electrode. It may be primary reference electrode like hydrogen electrode or secondary reference electrode like calomel electrode.

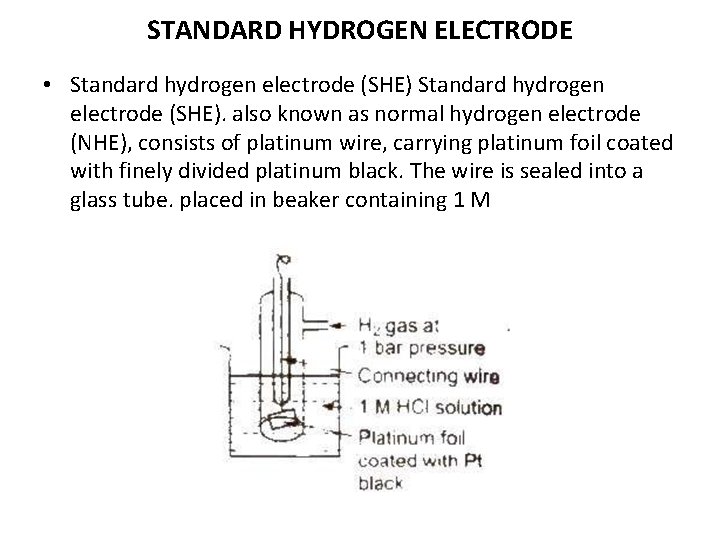
STANDARD HYDROGEN ELECTRODE • Standard hydrogen electrode (SHE). also known as normal hydrogen electrode (NHE), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. The wire is sealed into a glass tube. placed in beaker containing 1 M

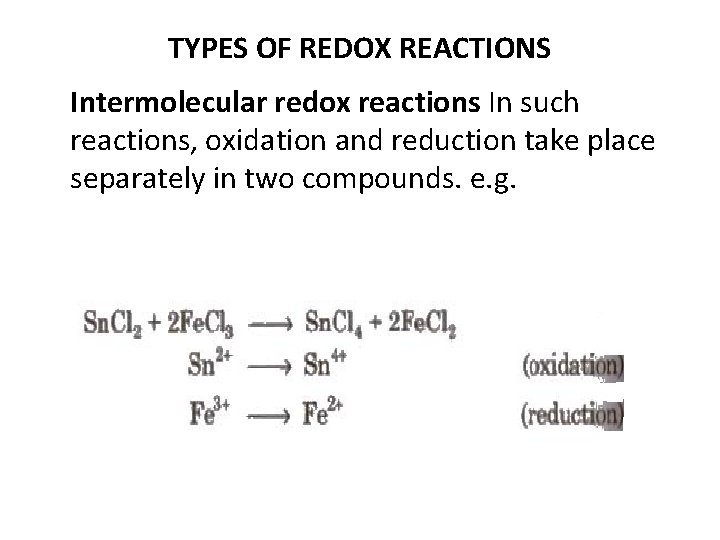
TYPES OF REDOX REACTIONS Intermolecular redox reactions In such reactions, oxidation and reduction take place separately in two compounds. e. g.
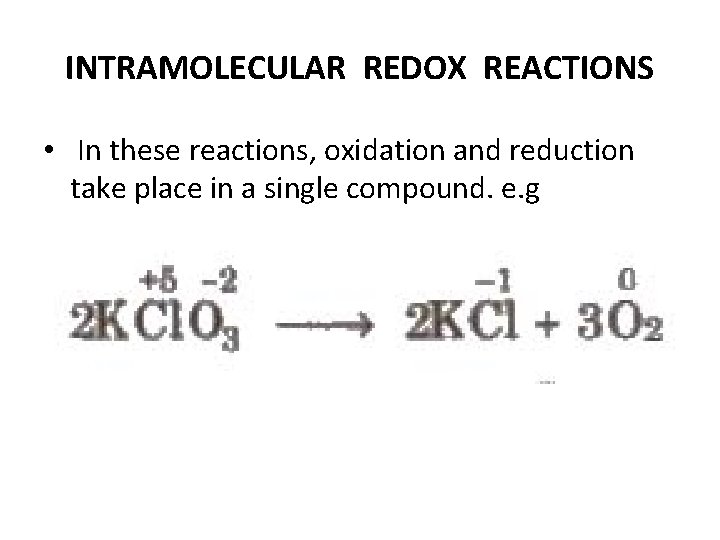
INTRAMOLECULAR REDOX REACTIONS • In these reactions, oxidation and reduction take place in a single compound. e. g

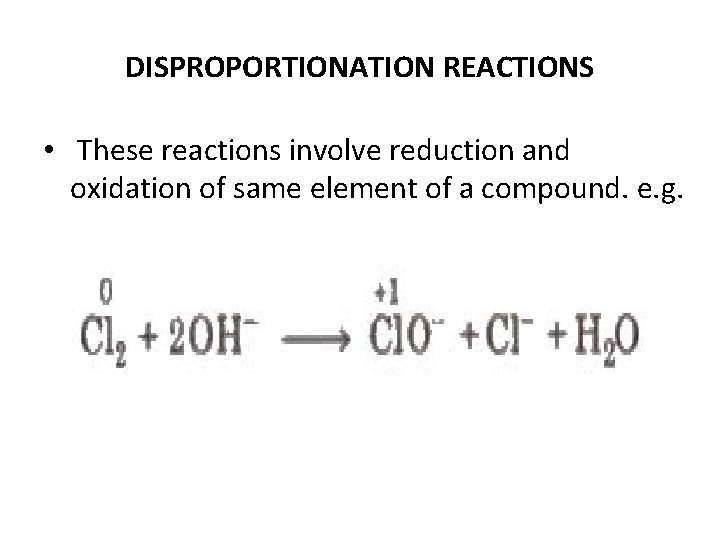
DISPROPORTIONATION REACTIONS • These reactions involve reduction and oxidation of same element of a compound. e. g.

BALANCING OF REDOX REACTION • OXIDATION NUMBER METHOD • ION ELECTRON METHOD

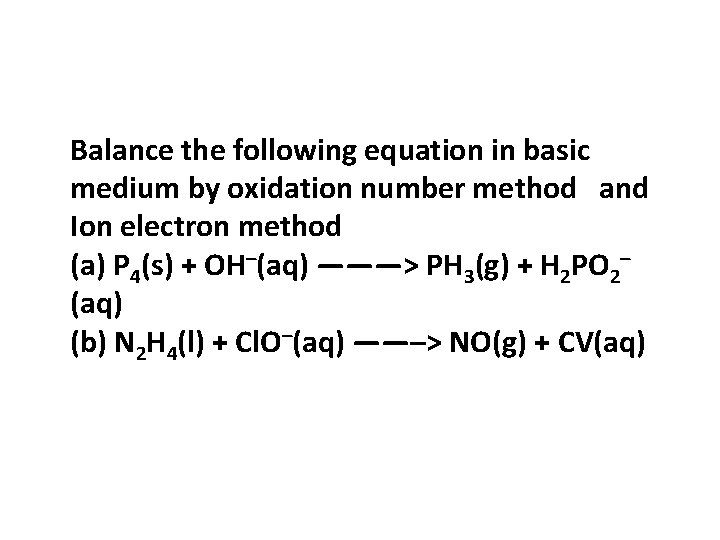
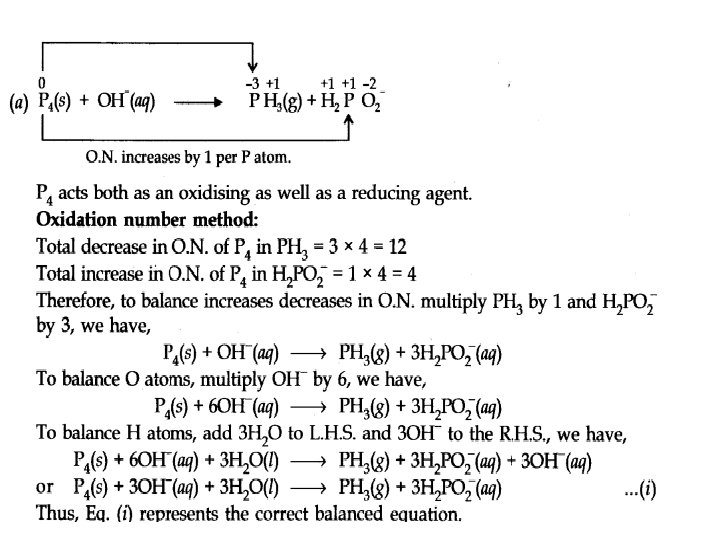
Balance the following equation in basic medium by oxidation number method and Ion electron method (a) P 4(s) + OH–(aq) ———> PH 3(g) + H 2 PO 2– (aq) (b) N 2 H 4(l) + Cl. O–(aq) ——–> NO(g) + CV(aq)


