REDOX OXIDATION REDUCTION Definitions OXIDATION GAIN OF OXYGEN









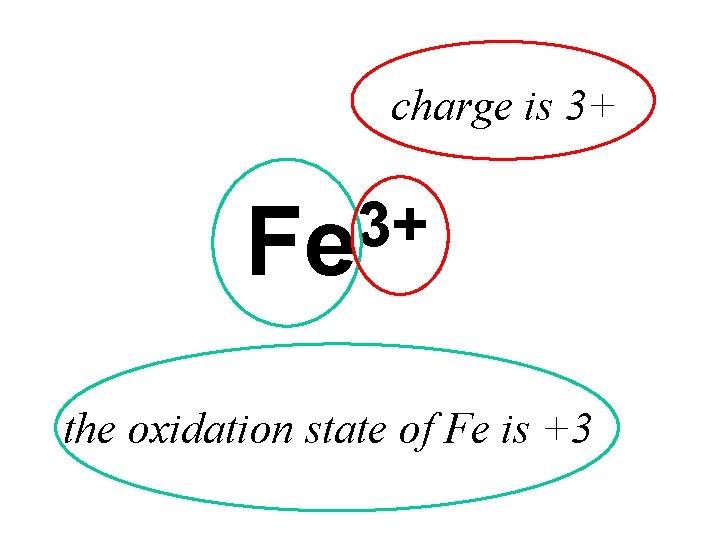





- Slides: 67

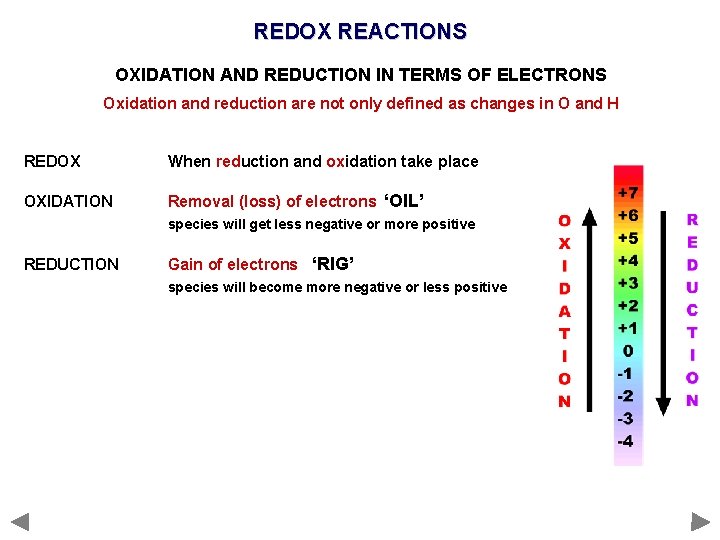
REDOX


OXIDATION & REDUCTION - Definitions OXIDATION GAIN OF OXYGEN 2 Mg + O 2 ——> 2 Mg. O magnesium has been oxidised as it has gained oxygen REMOVAL (LOSS) OF HYDROGEN C 2 H 5 OH ——> CH 3 CHO + H 2 ethanol has been oxidised as it has ‘lost’ hydrogen

OXIDATION & REDUCTION - Definitions REDUCTION GAIN OF HYDROGEN C 2 H 4 + H 2 ——> C 2 H 6 ethene has been reduced as it has gained hydrogen REMOVAL (LOSS) OF OXYGEN Cu. O + H 2 ——> Cu + H 2 O copper(II) oxide has been reduced as it has ‘lost’ oxygen However as chemistry became more sophisticated, it was realised that another definition was required

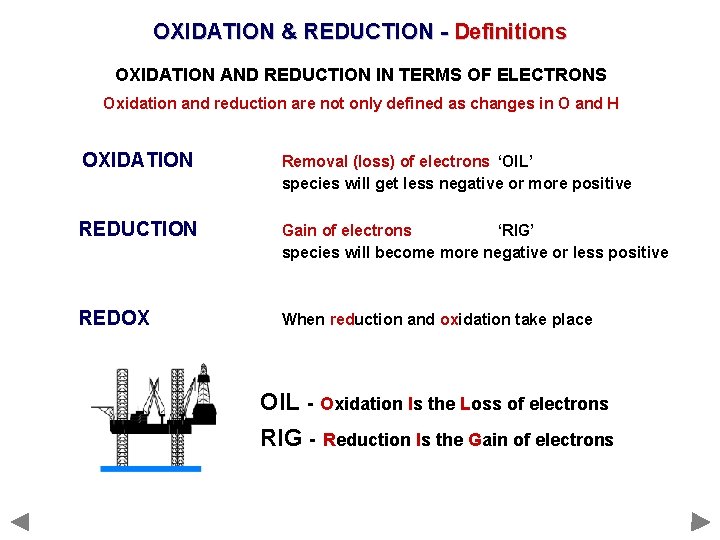
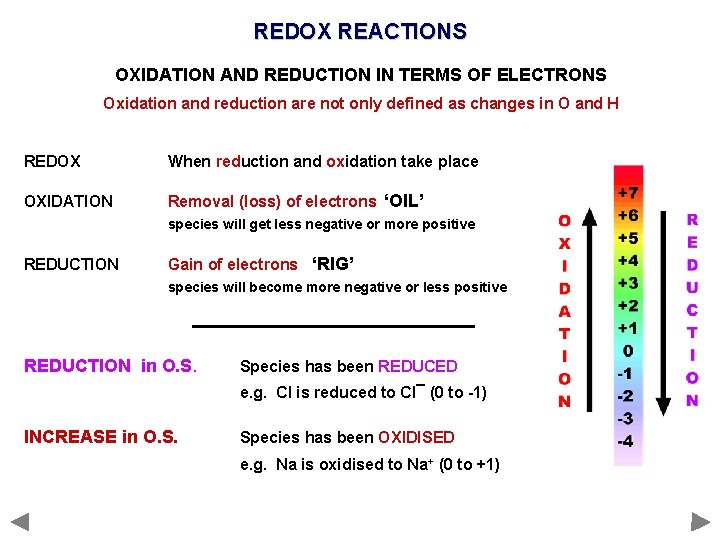
OXIDATION & REDUCTION - Definitions OXIDATION AND REDUCTION IN TERMS OF ELECTRONS Oxidation and reduction are not only defined as changes in O and H. . . OXIDATION Removal (loss) of electrons ‘OIL’ species will get less negative or more positive REDUCTION Gain of electrons ‘RIG’ species will become more negative or less positive REDOX When reduction and oxidation take place

OXIDATION & REDUCTION - Definitions OXIDATION AND REDUCTION IN TERMS OF ELECTRONS Oxidation and reduction are not only defined as changes in O and H. . . OXIDATION Removal (loss) of electrons ‘OIL’ species will get less negative or more positive REDUCTION Gain of electrons ‘RIG’ species will become more negative or less positive REDOX When reduction and oxidation take place OIL - Oxidation Is the Loss of electrons RIG - Reduction Is the Gain of electrons

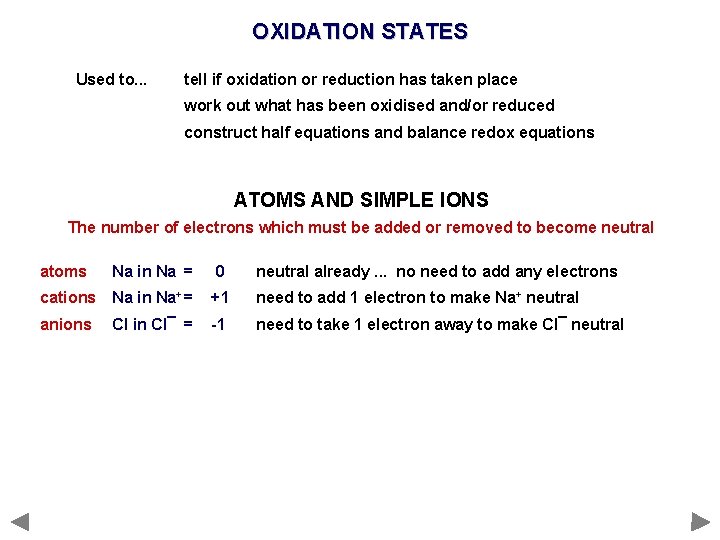
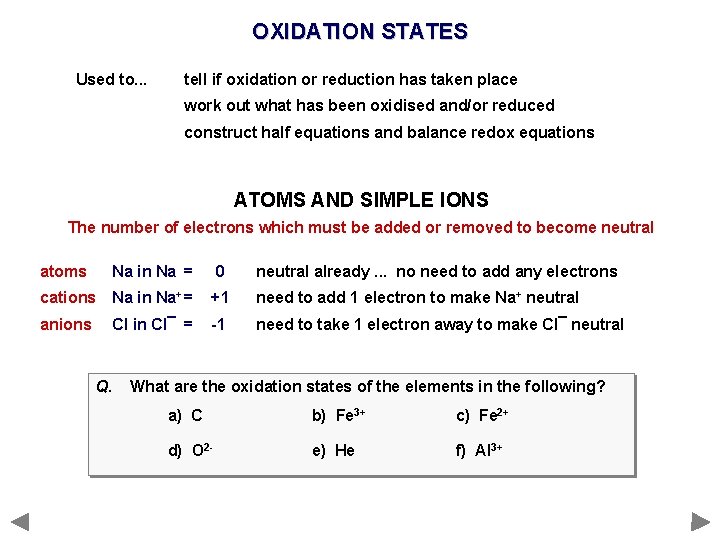
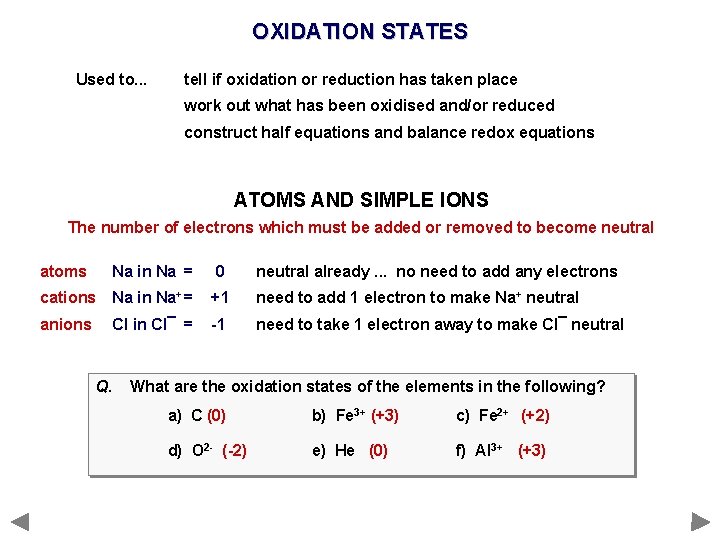
OXIDATION STATES Used to. . . tell if oxidation or reduction has taken place work out what has been oxidised and/or reduced construct half equations and balance redox equations ATOMS AND SIMPLE IONS The number of electrons which must be added or removed to become neutral atoms Na in Na = 0 neutral already. . . no need to add any electrons cations Na in Na+ = +1 need to add 1 electron to make Na+ neutral anions Cl in Cl¯ = -1 need to take 1 electron away to make Cl¯ neutral

OXIDATION STATES Used to. . . tell if oxidation or reduction has taken place work out what has been oxidised and/or reduced construct half equations and balance redox equations ATOMS AND SIMPLE IONS The number of electrons which must be added or removed to become neutral atoms Na in Na = 0 neutral already. . . no need to add any electrons cations Na in Na+ = +1 need to add 1 electron to make Na+ neutral anions Cl in Cl¯ = -1 need to take 1 electron away to make Cl¯ neutral Q. What are the oxidation states of the elements in the following? a) C b) Fe 3+ c) Fe 2+ d) O 2 - e) He f) Al 3+

OXIDATION STATES Used to. . . tell if oxidation or reduction has taken place work out what has been oxidised and/or reduced construct half equations and balance redox equations ATOMS AND SIMPLE IONS The number of electrons which must be added or removed to become neutral atoms Na in Na = 0 neutral already. . . no need to add any electrons cations Na in Na+ = +1 need to add 1 electron to make Na+ neutral anions Cl in Cl¯ = -1 need to take 1 electron away to make Cl¯ neutral Q. What are the oxidation states of the elements in the following? a) C (0) b) Fe 3+ (+3) c) Fe 2+ (+2) d) O 2 - (-2) e) He (0) f) Al 3+ (+3)

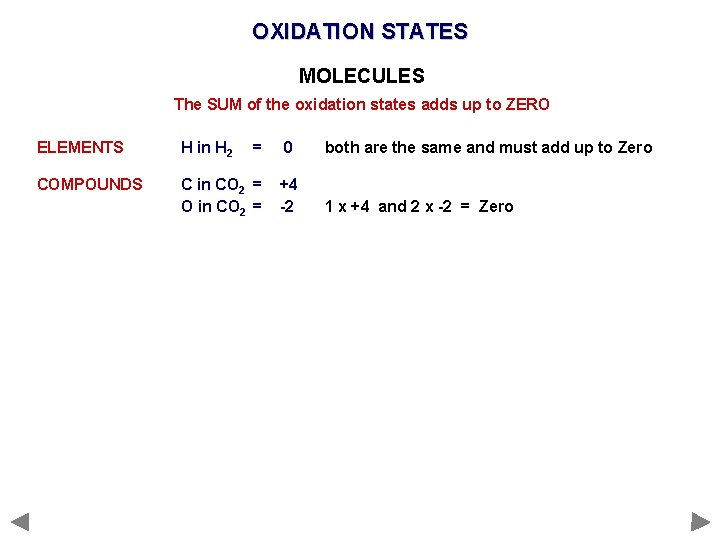
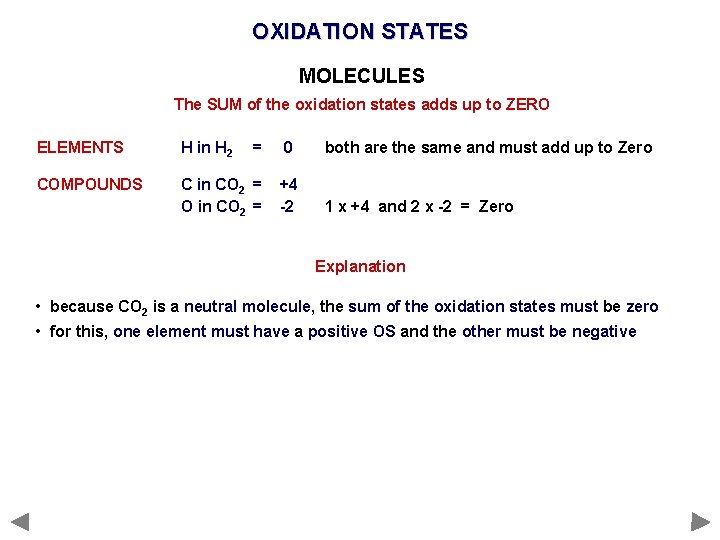
OXIDATION STATES MOLECULES The SUM of the oxidation states adds up to ZERO ELEMENTS H in H 2 = COMPOUNDS C in CO 2 = O in CO 2 = 0 both are the same and must add up to Zero +4 -2 1 x +4 and 2 x -2 = Zero

OXIDATION STATES MOLECULES The SUM of the oxidation states adds up to ZERO ELEMENTS H in H 2 = COMPOUNDS C in CO 2 = O in CO 2 = 0 both are the same and must add up to Zero +4 -2 1 x +4 and 2 x -2 = Zero Explanation • because CO 2 is a neutral molecule, the sum of the oxidation states must be zero • for this, one element must have a positive OS and the other must be negative

OXIDATION STATES MOLECULES The SUM of the oxidation states adds up to ZERO ELEMENTS H in H 2 = COMPOUNDS C in CO 2 = O in CO 2 = 0 both are the same and must add up to Zero +4 -2 1 x +4 and 2 x +2 = Zero HOW DO YOU DETERMINE WHICH IS THE POSITIVE ONE? • the more electronegative species will have the negative value • electronegativity increases across a period and decreases down a group • O is further to the right than C in the periodic table so it has the negative value

OXIDATION STATES MOLECULES The SUM of the oxidation states adds up to ZERO ELEMENTS H in H 2 = COMPOUNDS C in CO 2 = O in CO 2 = 0 both are the same and must add up to Zero +4 -2 1 x +4 and 2 x +2 = Zero HOW DO YOU DETERMINE THE VALUE OF AN ELEMENT’S OXIDATION STATE? • from its position in the periodic table and/or • the other element(s) present in the formula

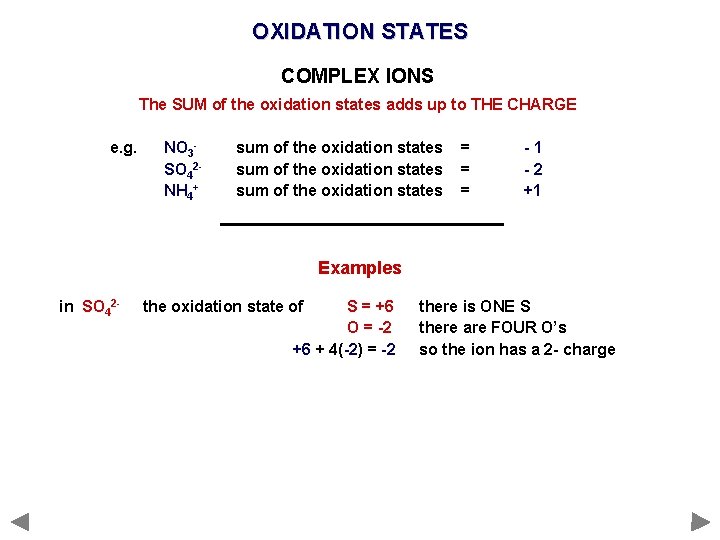
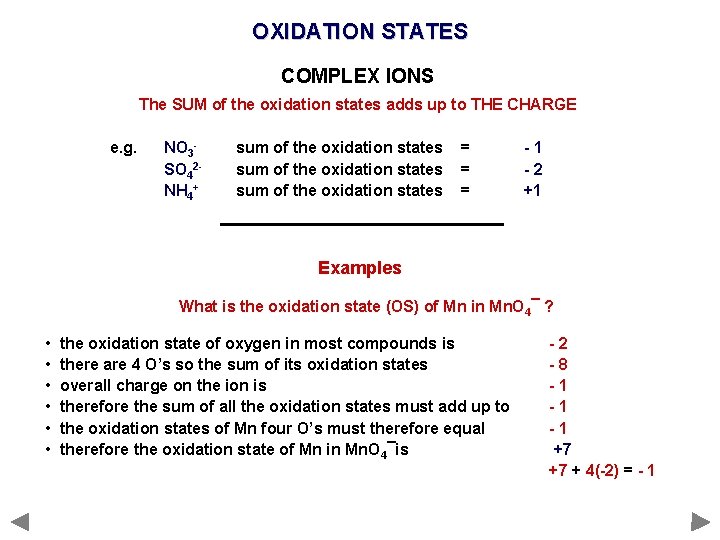
OXIDATION STATES COMPLEX IONS The SUM of the oxidation states adds up to THE CHARGE e. g. NO 3 SO 42 NH 4+ sum of the oxidation states = = = -1 -2 +1 Examples in SO 42 - the oxidation state of S = +6 O = -2 +6 + 4(-2) = -2 there is ONE S there are FOUR O’s so the ion has a 2 - charge

OXIDATION STATES COMPLEX IONS The SUM of the oxidation states adds up to THE CHARGE e. g. NO 3 SO 42 NH 4+ sum of the oxidation states = = = -1 -2 +1 Examples What is the oxidation state (OS) of Mn in Mn. O 4¯ ? • • • the oxidation state of oxygen in most compounds is there are 4 O’s so the sum of its oxidation states overall charge on the ion is therefore the sum of all the oxidation states must add up to the oxidation states of Mn four O’s must therefore equal therefore the oxidation state of Mn in Mn. O 4¯is -2 -8 -1 -1 -1 +7 +7 + 4(-2) = - 1

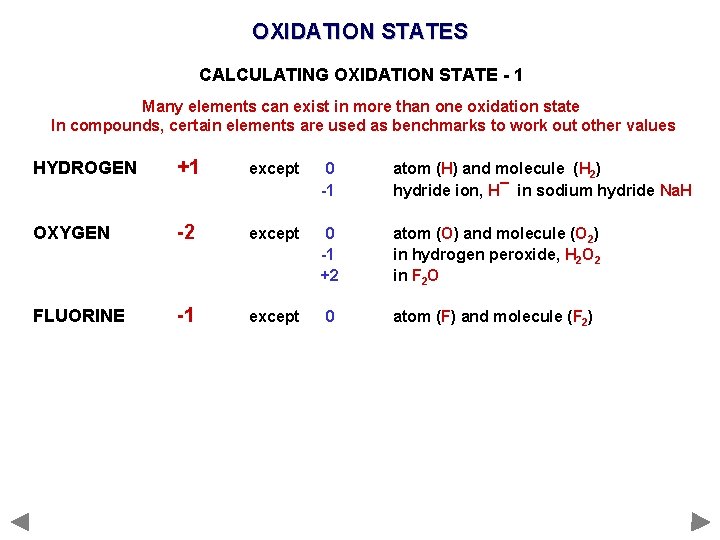
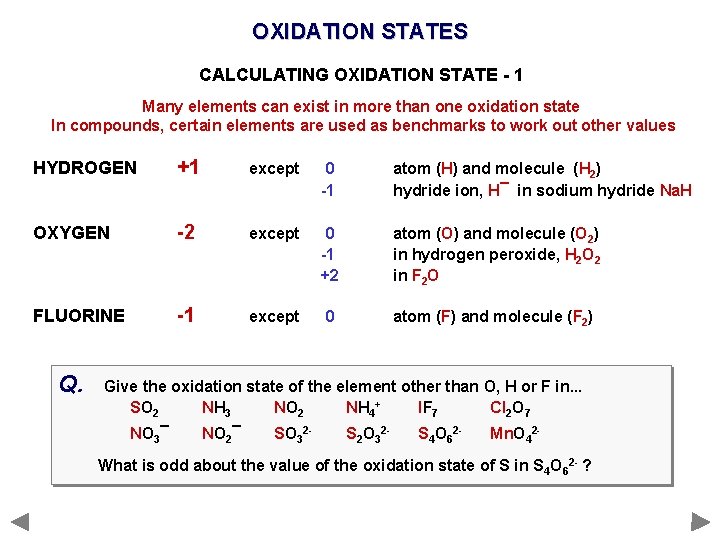
OXIDATION STATES CALCULATING OXIDATION STATE - 1 Many elements can exist in more than one oxidation state In compounds, certain elements are used as benchmarks to work out other values HYDROGEN +1 except 0 -1 atom (H) and molecule (H 2) hydride ion, H¯ in sodium hydride Na. H OXYGEN -2 except 0 -1 +2 atom (O) and molecule (O 2) in hydrogen peroxide, H 2 O 2 in F 2 O FLUORINE -1 except 0 atom (F) and molecule (F 2)


OXIDATION STATES CALCULATING OXIDATION STATE - 1 Many elements can exist in more than one oxidation state In compounds, certain elements are used as benchmarks to work out other values HYDROGEN +1 except 0 -1 atom (H) and molecule (H 2) hydride ion, H¯ in sodium hydride Na. H OXYGEN -2 except 0 -1 +2 atom (O) and molecule (O 2) in hydrogen peroxide, H 2 O 2 in F 2 O FLUORINE -1 except 0 atom (F) and molecule (F 2) Q. Give the oxidation state of the element other than O, H or F in. . . SO 2 NH 3 NO 2 NH 4+ IF 7 Cl 2 O 7 NO 3¯ NO 2¯ SO 32 - S 2 O 32 - S 4 O 62 - Mn. O 42 - What is odd about the value of the oxidation state of S in S 4 O 62 - ?

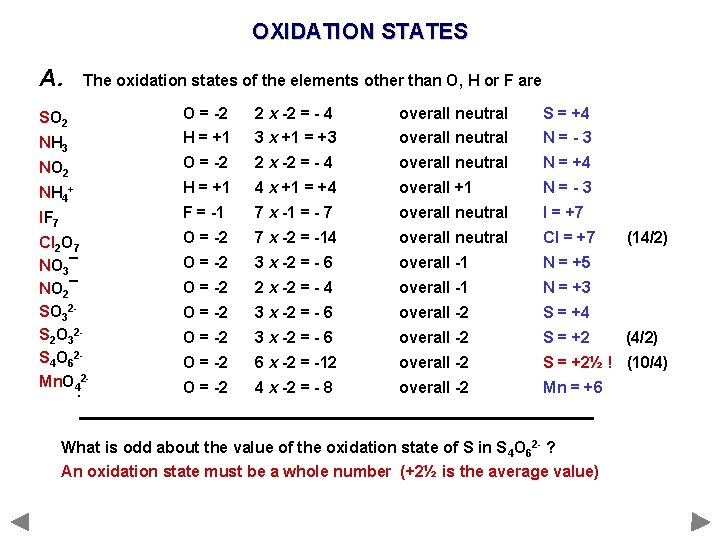
OXIDATION STATES A. The oxidation states of the elements other than O, H or F are SO 22 SO NH 3 NH O = -2 H = +1 2 x -2 = - 4 3 x +1 = +3 overall neutral S = +4 N=-3 NO 2 NH 4++ NH O = -2 2 x -2 = - 4 overall neutral N = +4 H = +1 4 x +1 = +4 overall +1 N=-3 IF 7 Cl 2 O O 7 Cl F = -1 7 x -1 = - 7 overall neutral I = +7 O = -2 7 x -2 = -14 overall neutral Cl = +7 NO 33¯ NO 2¯ SO 322 S 22 O 3322 S S 44 O O 6622 Mn. O 42 - O = -2 3 x -2 = - 6 overall -1 N = +5 O = -2 2 x -2 = - 4 overall -1 N = +3 O = -2 3 x -2 = - 6 overall -2 S = +4 O = -2 3 x -2 = - 6 overall -2 S = +2 O = -2 6 x -2 = -12 overall -2 S = +2½ ! (10/4) O = -2 4 x -2 = - 8 overall -2 Mn = +6 3 4 2 7 4 What is odd about the value of the oxidation state of S in S 4 O 62 - ? An oxidation state must be a whole number (+2½ is the average value) (14/2) (4/2)

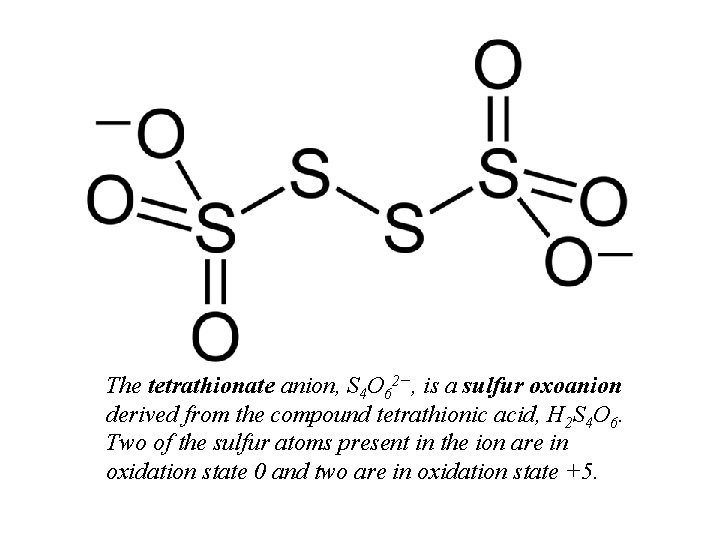
The tetrathionate anion, S 4 O 62−, is a sulfur oxoanion derived from the compound tetrathionic acid, H 2 S 4 O 6. Two of the sulfur atoms present in the ion are in oxidation state 0 and two are in oxidation state +5.

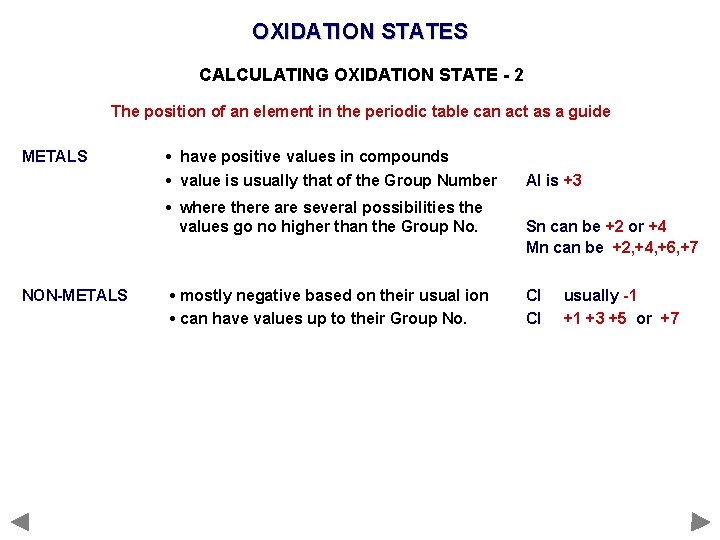
OXIDATION STATES CALCULATING OXIDATION STATE - 2 The position of an element in the periodic table can act as a guide METALS • have positive values in compounds • value is usually that of the Group Number • where there are several possibilities the values go no higher than the Group No. NON-METALS • mostly negative based on their usual ion • can have values up to their Group No. Al is +3 Sn can be +2 or +4 Mn can be +2, +4, +6, +7 Cl Cl usually -1 +1 +3 +5 or +7

OXIDATION STATES CALCULATING OXIDATION STATE - 2 The position of an element in the periodic table can act as a guide METALS • have positive values in compounds • value is usually that of the Group Number • where there are several possibilities the values go no higher than the Group No. NON-METALS Q. • mostly negative based on their usual ion • can have values up to their Group No. Al is +3 Sn can be +2 or +4 Mn can be +2, +4, +6, +7 Cl Cl usually -1 +1 +3 +5 or +7 What is theoretical maximum oxidation state of the following elements? Na P Ba Pb S Mn Cr What will be the usual and the maximum oxidation state in compounds of? Li Br Sr O B N +1

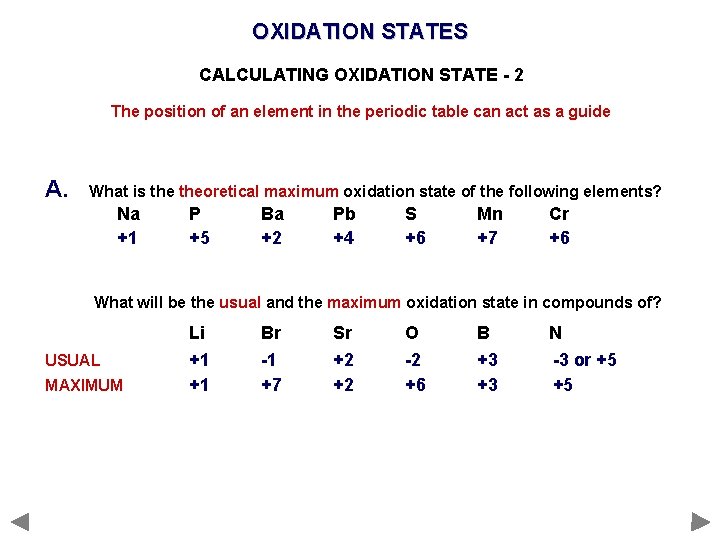
OXIDATION STATES CALCULATING OXIDATION STATE - 2 The position of an element in the periodic table can act as a guide A. What is theoretical maximum oxidation state of the following elements? Na +1 P +5 Ba +2 Pb +4 S +6 Mn +7 Cr +6 What will be the usual and the maximum oxidation state in compounds of? USUAL MAXIMUM Li +1 +1 Br -1 +7 Sr +2 +2 O -2 +6 B +3 +3 N -3 or +5 +5

OXIDATION STATES CALCULATING OXIDATION STATE - 2 Q. What is the oxidation state of each element in the following compounds/ions ? CH 4 PCl 3 NCl 3 CS 2 ICl 5 Br. F 3 PCl 4 H 3 PO 4 NH 4 Cl H 2 SO 4 Mg. CO 3 SOCl 2

OXIDATION STATES CALCULATING OXIDATION STATE - 2 Q. What is the oxidation state of each element in the following compounds/ions ? CH 4 C=-4 H = +1 PCl 3 P = +3 Cl = -1 NCl 3 N = +3 Cl = -1 CS 2 C = +4 S = -2 ICl 5 I = +5 Cl = -1 Br. F 3 Br = +3 F = -1 PCl 4 P = +4 Cl = -1 H 3 PO 4 P = +5 H = +1 O = -2 NH 4 Cl N = -3 H = +1 Cl = -1 H 2 SO 4 S = +6 H = +1 O = -2 Mg. CO 3 Mg = +2 C = +4 O = -2 SOCl 2 S = +4 O = -2 Cl = -1

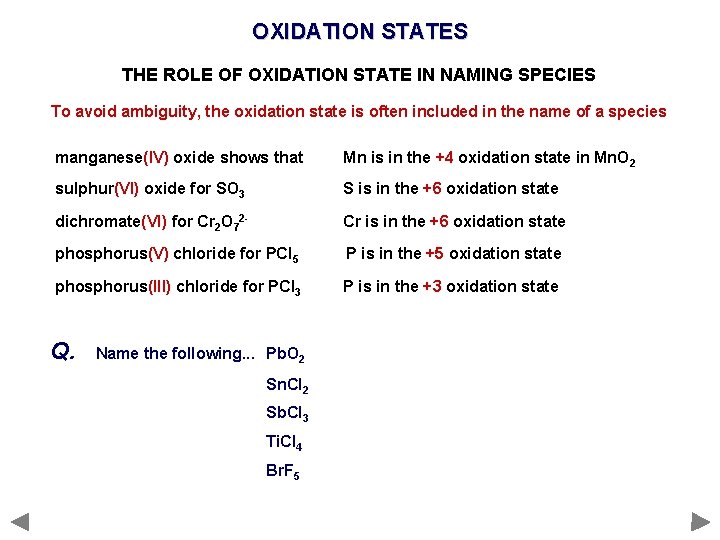
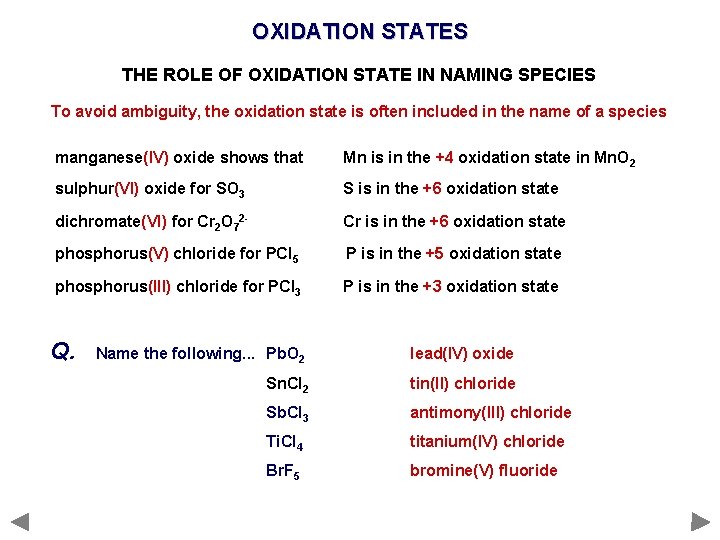
OXIDATION STATES THE ROLE OF OXIDATION STATE IN NAMING SPECIES To avoid ambiguity, the oxidation state is often included in the name of a species manganese(IV) oxide shows that Mn is in the +4 oxidation state in Mn. O 2 sulphur(VI) oxide for SO 3 S is in the +6 oxidation state dichromate(VI) for Cr 2 O 72 - Cr is in the +6 oxidation state phosphorus(V) chloride for PCl 5 P is in the +5 oxidation state phosphorus(III) chloride for PCl 3 P is in the +3 oxidation state Q. Name the following. . . Pb. O 2 Sn. Cl 2 Sb. Cl 3 Ti. Cl 4 Br. F 5

OXIDATION STATES THE ROLE OF OXIDATION STATE IN NAMING SPECIES To avoid ambiguity, the oxidation state is often included in the name of a species manganese(IV) oxide shows that Mn is in the +4 oxidation state in Mn. O 2 sulphur(VI) oxide for SO 3 S is in the +6 oxidation state dichromate(VI) for Cr 2 O 72 - Cr is in the +6 oxidation state phosphorus(V) chloride for PCl 5 P is in the +5 oxidation state phosphorus(III) chloride for PCl 3 P is in the +3 oxidation state Q. Name the following. . . Pb. O 2 lead(IV) oxide Sn. Cl 2 tin(II) chloride Sb. Cl 3 antimony(III) chloride Ti. Cl 4 titanium(IV) chloride Br. F 5 bromine(V) fluoride


Now try question - 1, 2, 3, 4 in the text book ( Oxidation number – Pages 30 & 31 )





REDOX REACTIONS OXIDATION AND REDUCTION IN TERMS OF ELECTRONS Oxidation and reduction are not only defined as changes in O and H REDOX When reduction and oxidation take place OXIDATION Removal (loss) of electrons ‘OIL’ species will get less negative or more positive REDUCTION Gain of electrons ‘RIG’ species will become more negative or less positive

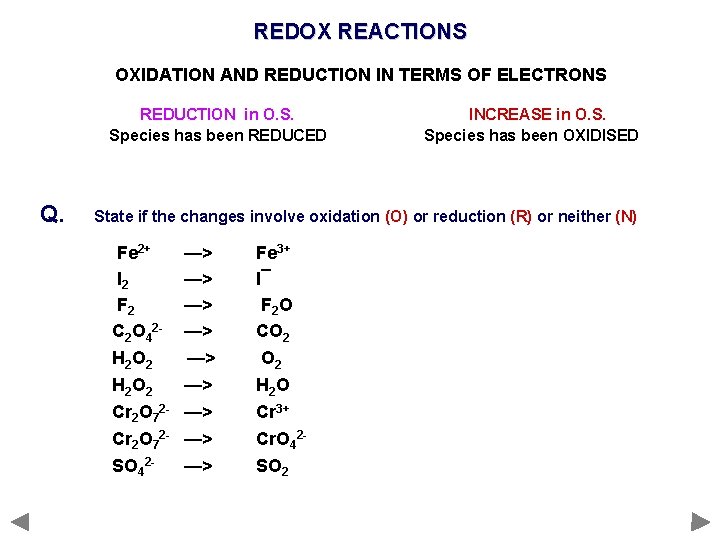
REDOX REACTIONS OXIDATION AND REDUCTION IN TERMS OF ELECTRONS Oxidation and reduction are not only defined as changes in O and H REDOX When reduction and oxidation take place OXIDATION Removal (loss) of electrons ‘OIL’ species will get less negative or more positive REDUCTION Gain of electrons ‘RIG’ species will become more negative or less positive REDUCTION in O. S. Species has been REDUCED e. g. Cl is reduced to Cl¯ (0 to -1) INCREASE in O. S. Species has been OXIDISED e. g. Na is oxidised to Na+ (0 to +1)

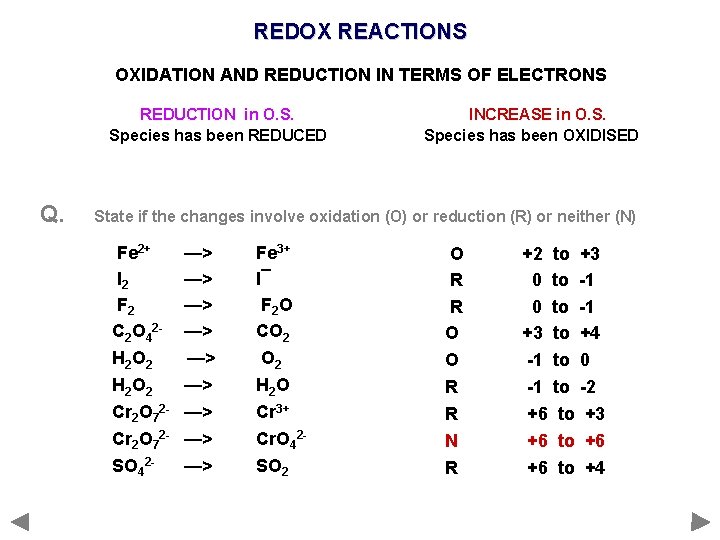
REDOX REACTIONS OXIDATION AND REDUCTION IN TERMS OF ELECTRONS REDUCTION in O. S. Species has been REDUCED Q. INCREASE in O. S. Species has been OXIDISED State if the changes involve oxidation (O) or reduction (R) or neither (N) Fe 2+ I 2 F 2 C 2 O 42 H 2 O 2 Cr 2 O 72 SO 42 - —> —> —> Fe 3+ I¯ F 2 O CO 2 H 2 O Cr 3+ Cr. O 42 SO 2

REDOX REACTIONS OXIDATION AND REDUCTION IN TERMS OF ELECTRONS REDUCTION in O. S. Species has been REDUCED Q. INCREASE in O. S. Species has been OXIDISED State if the changes involve oxidation (O) or reduction (R) or neither (N) Fe 2+ I 2 F 2 C 2 O 42 H 2 O 2 Cr 2 O 72 SO 42 - —> —> —> Fe 3+ I¯ F 2 O CO 2 H 2 O Cr 3+ Cr. O 42 SO 2 O R R O O R R N R O +2 to+2+3 to +3 R 0 to 0 -1 to -1 O +3 to+3+4 to +4 O -1 to -10 to 0 R -1 to -1 -2 to -2 R +6 to+6+3 to +3 N +6 to+6+6 to +6 R +6 to+6+4 to +4
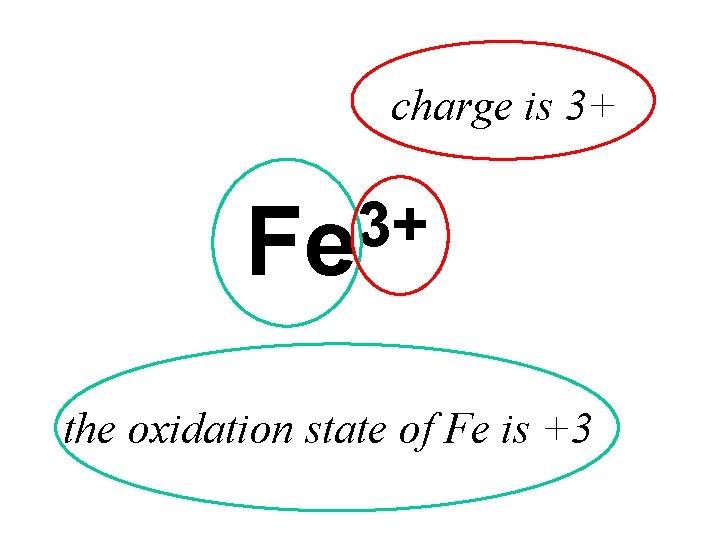
charge is 3+ 3+ Fe the oxidation state of Fe is +3

Stop here !!!

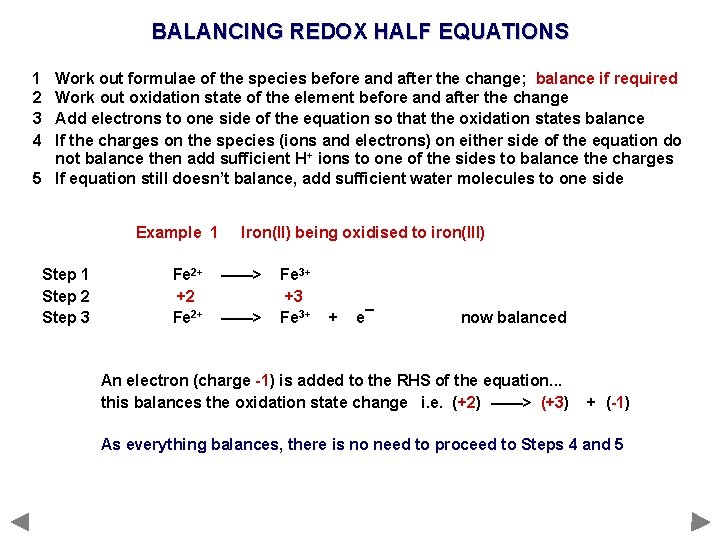
BALANCING REDOX HALF EQUATIONS 1 2 3 4 Work out formulae of the species before and after the change; balance if required Work out oxidation state of the element before and after the change Add electrons to one side of the equation so that the oxidation states balance If the charges on the species (ions and electrons) on either side of the equation do not balance then add sufficient H+ ions to one of the sides to balance the charges 5 If equation still doesn’t balance, add sufficient water molecules to one side

BALANCING REDOX HALF EQUATIONS 1 2 3 4 Work out formulae of the species before and after the change; balance if required Work out oxidation state of the element before and after the change Add electrons to one side of the equation so that the oxidation states balance If the charges on the species (ions and electrons) on either side of the equation do not balance then add sufficient H+ ions to one of the sides to balance the charges 5 If equation still doesn’t balance, add sufficient water molecules to one side Example 1 Step 2 Step 3 Fe 2+ +2 Fe 2+ Iron(II) being oxidised to iron(III) ——> Fe 3+ +3 Fe 3+ + e¯ now balanced An electron (charge -1) is added to the RHS of the equation. . . this balances the oxidation state change i. e. (+2) ——> (+3) + (-1) As everything balances, there is no need to proceed to Steps 4 and 5

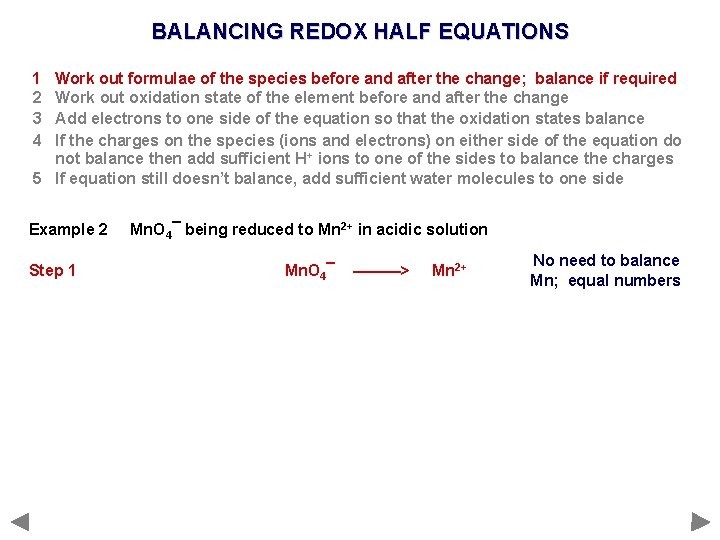
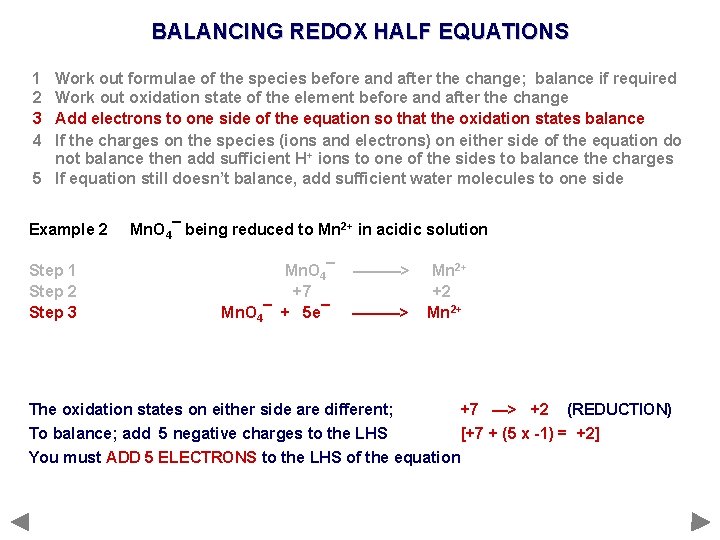
BALANCING REDOX HALF EQUATIONS 1 2 3 4 Work out formulae of the species before and after the change; balance if required Work out oxidation state of the element before and after the change Add electrons to one side of the equation so that the oxidation states balance If the charges on the species (ions and electrons) on either side of the equation do not balance then add sufficient H+ ions to one of the sides to balance the charges 5 If equation still doesn’t balance, add sufficient water molecules to one side Example 2 Mn. O 4¯ being reduced to Mn 2+ in acidic solution

BALANCING REDOX HALF EQUATIONS 1 2 3 4 Work out formulae of the species before and after the change; balance if required Work out oxidation state of the element before and after the change Add electrons to one side of the equation so that the oxidation states balance If the charges on the species (ions and electrons) on either side of the equation do not balance then add sufficient H+ ions to one of the sides to balance the charges 5 If equation still doesn’t balance, add sufficient water molecules to one side Example 2 Step 1 Mn. O 4¯ being reduced to Mn 2+ in acidic solution Mn. O 4¯ ———> Mn 2+ No need to balance Mn; equal numbers

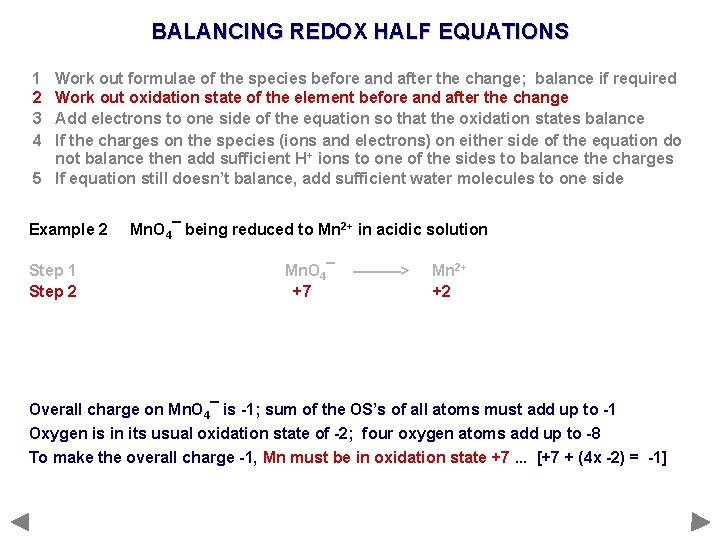
BALANCING REDOX HALF EQUATIONS 1 2 3 4 Work out formulae of the species before and after the change; balance if required Work out oxidation state of the element before and after the change Add electrons to one side of the equation so that the oxidation states balance If the charges on the species (ions and electrons) on either side of the equation do not balance then add sufficient H+ ions to one of the sides to balance the charges 5 If equation still doesn’t balance, add sufficient water molecules to one side Example 2 Step 1 Step 2 Mn. O 4¯ being reduced to Mn 2+ in acidic solution Mn. O 4¯ +7 ———> Mn 2+ +2 Overall charge on Mn. O 4¯ is -1; sum of the OS’s of all atoms must add up to -1 Oxygen is in its usual oxidation state of -2; four oxygen atoms add up to -8 To make the overall charge -1, Mn must be in oxidation state +7. . . [+7 + (4 x -2) = -1]

BALANCING REDOX HALF EQUATIONS 1 2 3 4 Work out formulae of the species before and after the change; balance if required Work out oxidation state of the element before and after the change Add electrons to one side of the equation so that the oxidation states balance If the charges on the species (ions and electrons) on either side of the equation do not balance then add sufficient H+ ions to one of the sides to balance the charges 5 If equation still doesn’t balance, add sufficient water molecules to one side Example 2 Step 1 Step 2 Step 3 Mn. O 4¯ being reduced to Mn 2+ in acidic solution Mn. O 4¯ +7 Mn. O 4¯ + 5 e¯ ———> Mn 2+ +2 Mn 2+ The oxidation states on either side are different; +7 —> +2 (REDUCTION) To balance; add 5 negative charges to the LHS [+7 + (5 x -1) = +2] You must ADD 5 ELECTRONS to the LHS of the equation

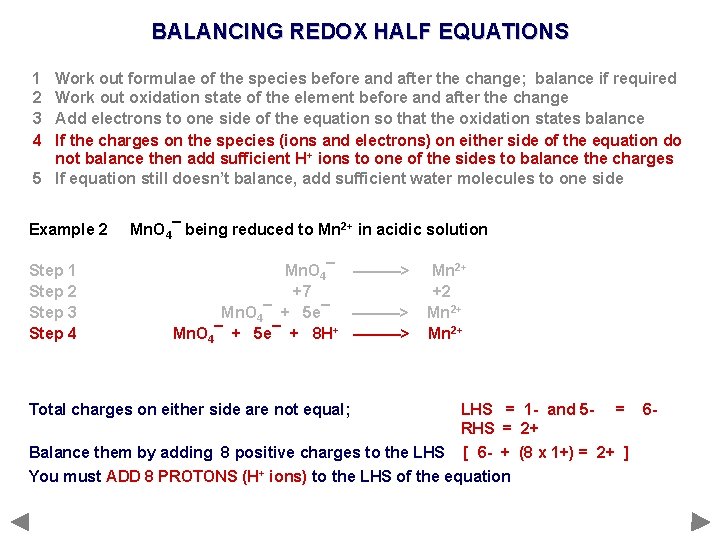
BALANCING REDOX HALF EQUATIONS 1 2 3 4 Work out formulae of the species before and after the change; balance if required Work out oxidation state of the element before and after the change Add electrons to one side of the equation so that the oxidation states balance If the charges on the species (ions and electrons) on either side of the equation do not balance then add sufficient H+ ions to one of the sides to balance the charges 5 If equation still doesn’t balance, add sufficient water molecules to one side Example 2 Step 1 Step 2 Step 3 Step 4 Mn. O 4¯ being reduced to Mn 2+ in acidic solution Mn. O 4¯ ———> +7 Mn. O 4¯ + 5 e¯ ———> Mn. O 4¯ + 5 e¯ + 8 H+ ———> Mn 2+ +2 Mn 2+ Total charges on either side are not equal; LHS = 1 - and 5 RHS = 2+ Balance them by adding 8 positive charges to the LHS [ 6 - + (8 x 1+) = 2+ ] You must ADD 8 PROTONS (H+ ions) to the LHS of the equation = 6 -

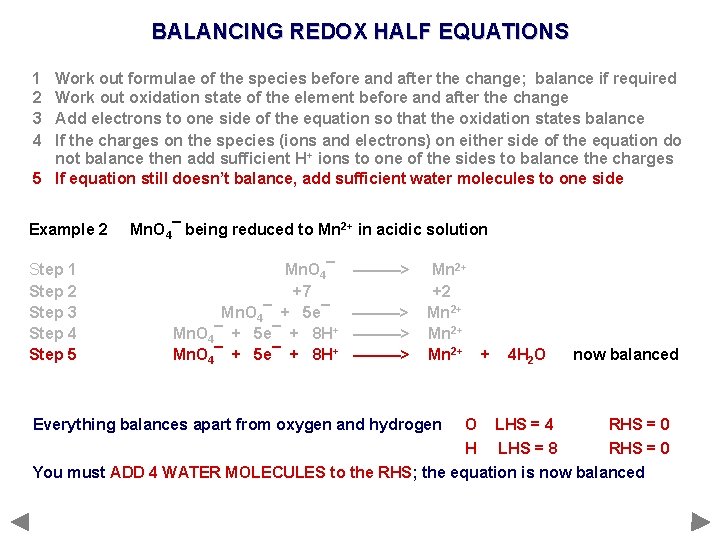
BALANCING REDOX HALF EQUATIONS 1 2 3 4 Work out formulae of the species before and after the change; balance if required Work out oxidation state of the element before and after the change Add electrons to one side of the equation so that the oxidation states balance If the charges on the species (ions and electrons) on either side of the equation do not balance then add sufficient H+ ions to one of the sides to balance the charges 5 If equation still doesn’t balance, add sufficient water molecules to one side Example 2 Step 1 Step 2 Step 3 Step 4 Step 5 Mn. O 4¯ being reduced to Mn 2+ in acidic solution Mn. O 4¯ +7 Mn. O 4¯ + 5 e¯ + 8 H+ ———> Mn 2+ +2 Mn 2+ + Everything balances apart from oxygen and hydrogen 4 H 2 O now balanced O LHS = 4 RHS = 0 H LHS = 8 RHS = 0 You must ADD 4 WATER MOLECULES to the RHS; the equation is now balanced

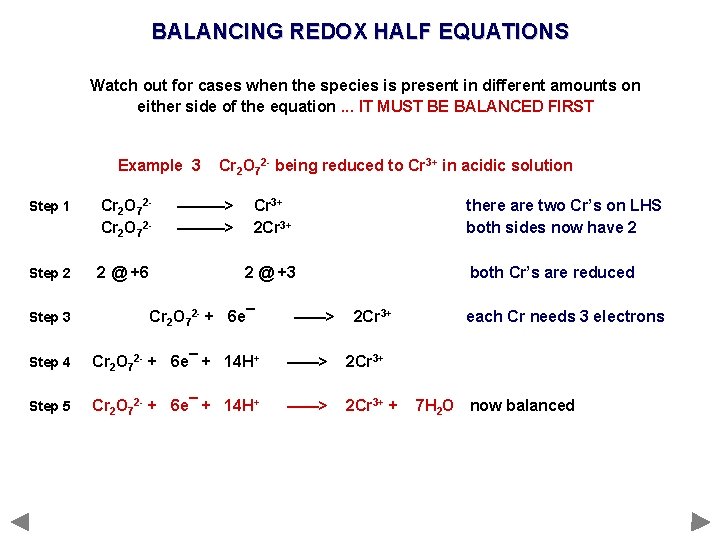
BALANCING REDOX HALF EQUATIONS Watch out for cases when the species is present in different amounts on either side of the equation. . . IT MUST BE BALANCED FIRST Example 3 Step 1 Cr 2 O 72 - Step 2 2 @ +6 Step 3 Cr 2 O 72 - being reduced to Cr 3+ in acidic solution ———> Cr 3+ 2 Cr 3+ there are two Cr’s on LHS both sides now have 2 2 @ +3 Cr 2 O 72 - + 6 e¯ ——> both Cr’s are reduced 2 Cr 3+ Step 4 Cr 2 O 72 - + 6 e¯ + 14 H+ ——> 2 Cr 3+ Step 5 Cr 2 O 72 - + 6 e¯ + 14 H+ ——> 2 Cr 3+ + each Cr needs 3 electrons 7 H 2 O now balanced

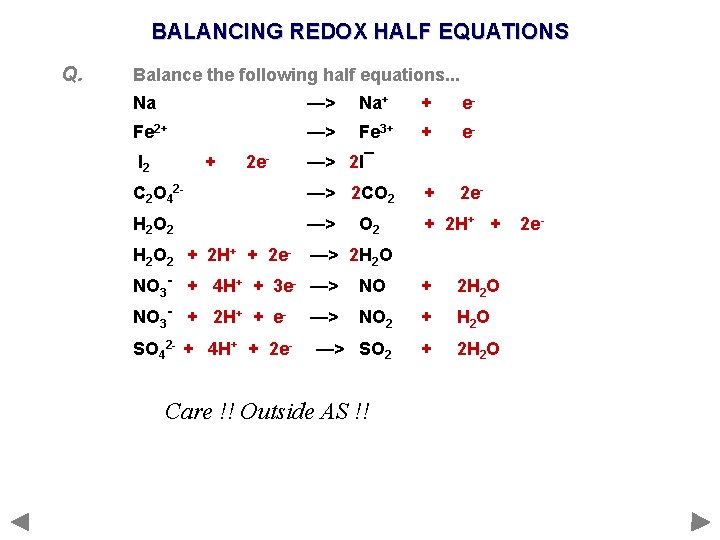
BALANCING REDOX HALF EQUATIONS Q. 1 2 3 4 5 Balance the following half equations. . . Na —> Na+ Fe 2+ —> Fe 3+ I 2 —> I¯ C 2 O 42 - —> CO 2 H 2 O 2 —> H 2 O NO 3 - —> NO 2 SO 42 - —> SO 2 REMINDER Work out the formula of the species before and after the change; balance if required Work out the oxidation state of the element before and after the change Add electrons to one side of the equation so that the oxidation states balance If the charges on all the species (ions and electrons) on either side of the equation do not balance then add sufficient H+ ions to one of the sides to balance the charges If the equation still doesn’t balance, add sufficient water molecules to one side

BALANCING REDOX HALF EQUATIONS Q. Balance the following half equations. . . Na —> Na+ + e- Fe 2+ —> Fe 3+ + e 2 e- I 2 + 2 e- —> 2 I¯ C 2 O 42 - —> 2 CO 2 + H 2 O 2 —> + 2 H+ + H 2 O 2 + 2 H+ + 2 e- —> 2 H 2 O O 2 NO 3 - + 4 H+ + 3 e- —> NO + 2 H 2 O NO 3 - + 2 H+ + e- NO 2 + H 2 O —> SO 2 + 2 H 2 O SO 42 - + 4 H+ + 2 e- —> Care !! Outside AS !! 2 e-

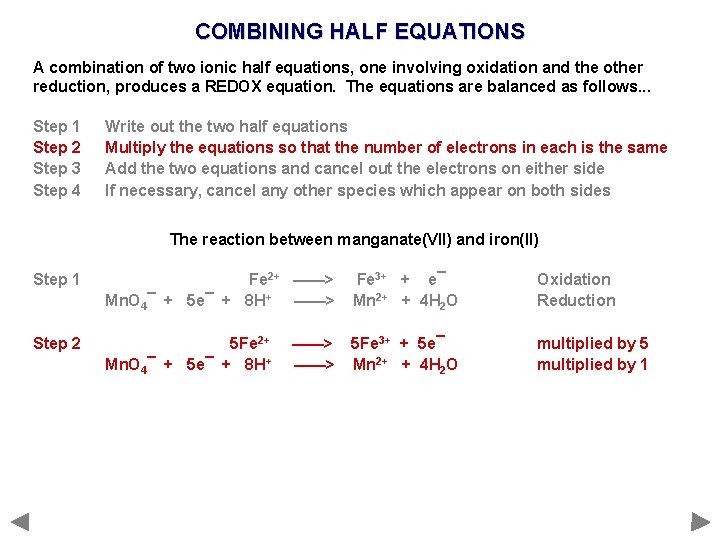
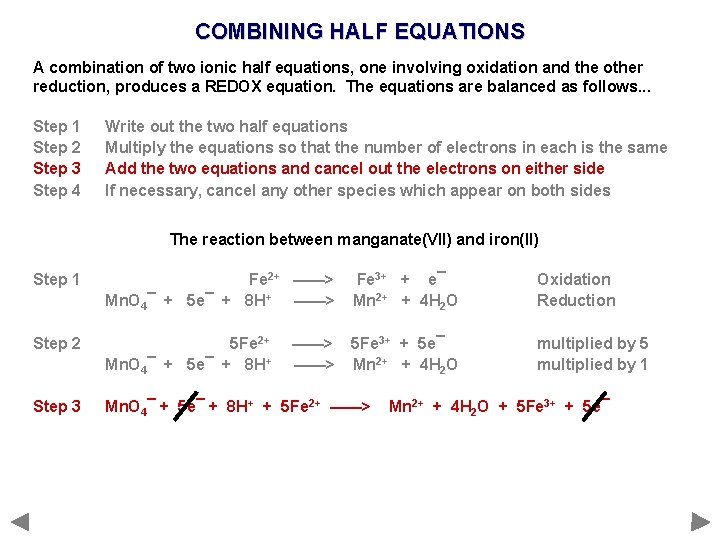
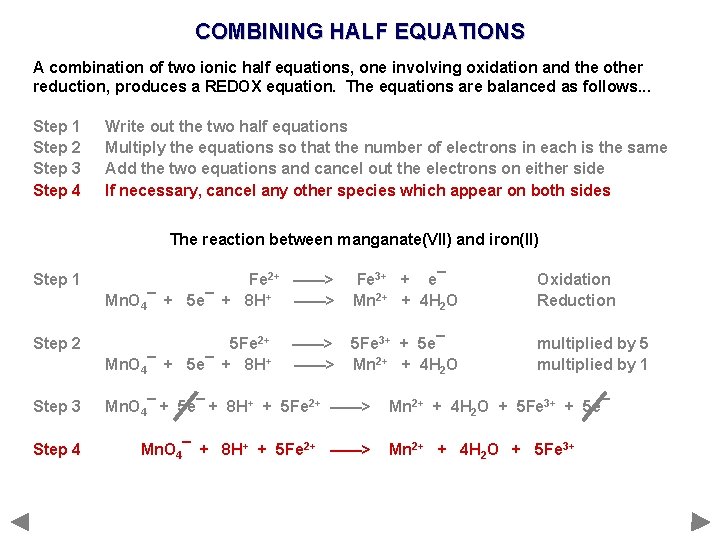
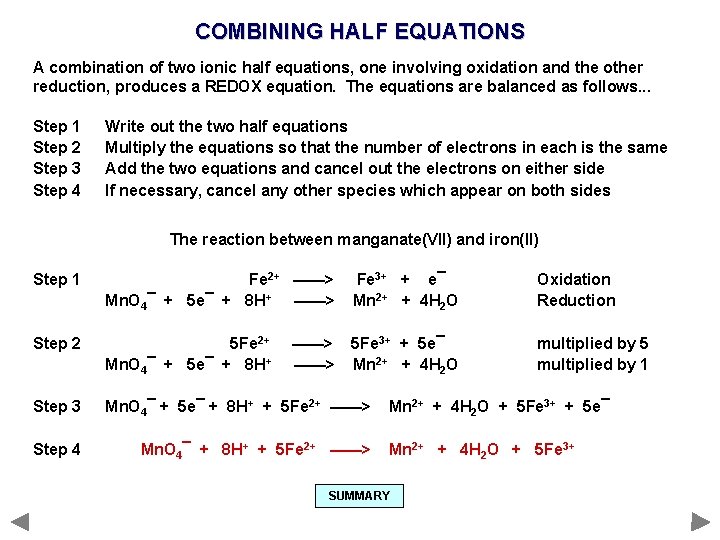
COMBINING HALF EQUATIONS A combination of two ionic half equations, one involving oxidation and the other reduction, produces a REDOX equation. The equations are balanced as follows. . . Step 1 Step 2 Step 3 Step 4 Write out the two half equations Multiply the equations so that the number of electrons in each is the same Add the two equations and cancel out the electrons on either side If necessary, cancel any other species which appear on both sides

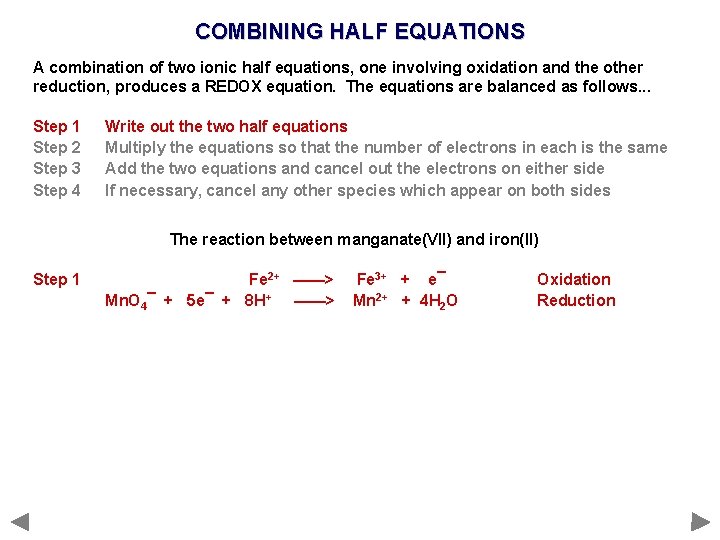
COMBINING HALF EQUATIONS A combination of two ionic half equations, one involving oxidation and the other reduction, produces a REDOX equation. The equations are balanced as follows. . . Step 1 Step 2 Step 3 Step 4 Write out the two half equations Multiply the equations so that the number of electrons in each is the same Add the two equations and cancel out the electrons on either side If necessary, cancel any other species which appear on both sides The reaction between manganate(VII) and iron(II)

COMBINING HALF EQUATIONS A combination of two ionic half equations, one involving oxidation and the other reduction, produces a REDOX equation. The equations are balanced as follows. . . Step 1 Step 2 Step 3 Step 4 Write out the two half equations Multiply the equations so that the number of electrons in each is the same Add the two equations and cancel out the electrons on either side If necessary, cancel any other species which appear on both sides The reaction between manganate(VII) and iron(II) Step 1 Fe 2+ ——> Mn. O 4¯ + 5 e¯ + 8 H+ ——> Fe 3+ + e¯ Mn 2+ + 4 H 2 O Oxidation Reduction

COMBINING HALF EQUATIONS A combination of two ionic half equations, one involving oxidation and the other reduction, produces a REDOX equation. The equations are balanced as follows. . . Step 1 Step 2 Step 3 Step 4 Write out the two half equations Multiply the equations so that the number of electrons in each is the same Add the two equations and cancel out the electrons on either side If necessary, cancel any other species which appear on both sides The reaction between manganate(VII) and iron(II) Step 1 Fe 2+ ——> Mn. O 4¯ + 5 e¯ + 8 H+ ——> Fe 3+ + e¯ Mn 2+ + 4 H 2 O Oxidation Reduction Step 2 5 Fe 2+ Mn. O 4¯ + 5 e¯ + 8 H+ 5 Fe 3+ + 5 e¯ Mn 2+ + 4 H 2 O multiplied by 5 multiplied by 1 ——>

COMBINING HALF EQUATIONS A combination of two ionic half equations, one involving oxidation and the other reduction, produces a REDOX equation. The equations are balanced as follows. . . Step 1 Step 2 Step 3 Step 4 Write out the two half equations Multiply the equations so that the number of electrons in each is the same Add the two equations and cancel out the electrons on either side If necessary, cancel any other species which appear on both sides The reaction between manganate(VII) and iron(II) Step 1 Fe 2+ ——> Mn. O 4¯ + 5 e¯ + 8 H+ ——> Fe 3+ + e¯ Mn 2+ + 4 H 2 O Oxidation Reduction Step 2 5 Fe 2+ Mn. O 4¯ + 5 e¯ + 8 H+ 5 Fe 3+ + 5 e¯ Mn 2+ + 4 H 2 O multiplied by 5 multiplied by 1 Step 3 Mn. O 4¯ + 5 e¯ + 8 H+ + 5 Fe 2+ ——> ——> Mn 2+ + 4 H 2 O + 5 Fe 3+ + 5 e¯

COMBINING HALF EQUATIONS A combination of two ionic half equations, one involving oxidation and the other reduction, produces a REDOX equation. The equations are balanced as follows. . . Step 1 Step 2 Step 3 Step 4 Write out the two half equations Multiply the equations so that the number of electrons in each is the same Add the two equations and cancel out the electrons on either side If necessary, cancel any other species which appear on both sides The reaction between manganate(VII) and iron(II) Step 1 Fe 2+ ——> Mn. O 4¯ + 5 e¯ + 8 H+ ——> Fe 3+ + e¯ Mn 2+ + 4 H 2 O Oxidation Reduction Step 2 5 Fe 2+ Mn. O 4¯ + 5 e¯ + 8 H+ 5 Fe 3+ + 5 e¯ Mn 2+ + 4 H 2 O multiplied by 5 multiplied by 1 Step 3 Mn. O 4¯ + 5 e¯ + 8 H+ + 5 Fe 2+ ——> Step 4 Mn. O 4¯ + 8 H+ + 5 Fe 2+ ——> ——> Mn 2+ + 4 H 2 O + 5 Fe 3+ + 5 e¯ Mn 2+ + 4 H 2 O + 5 Fe 3+

COMBINING HALF EQUATIONS A combination of two ionic half equations, one involving oxidation and the other reduction, produces a REDOX equation. The equations are balanced as follows. . . Step 1 Step 2 Step 3 Step 4 Write out the two half equations Multiply the equations so that the number of electrons in each is the same Add the two equations and cancel out the electrons on either side If necessary, cancel any other species which appear on both sides The reaction between manganate(VII) and iron(II) Step 1 Fe 2+ ——> Mn. O 4¯ + 5 e¯ + 8 H+ ——> Fe 3+ + e¯ Mn 2+ + 4 H 2 O Oxidation Reduction Step 2 5 Fe 2+ Mn. O 4¯ + 5 e¯ + 8 H+ 5 Fe 3+ + 5 e¯ Mn 2+ + 4 H 2 O multiplied by 5 multiplied by 1 Step 3 Mn. O 4¯ + 5 e¯ + 8 H+ + 5 Fe 2+ ——> Step 4 Mn. O 4¯ + 8 H+ + 5 Fe 2+ ——> ——> Mn 2+ + 4 H 2 O + 5 Fe 3+ + 5 e¯ Mn 2+ + 4 H 2 O + 5 Fe 3+ SUMMARY

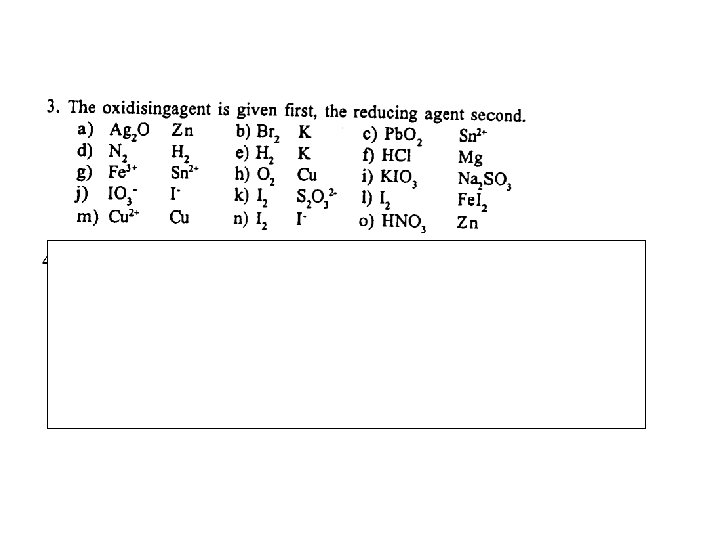
COMBINING HALF EQUATIONS A combination of two ionic half equations, one involving oxidation and the other reduction, produces a REDOX equation. The equations are balanced as follows. . . Step 1 Step 2 Step 3 Step 4 Q. Write out the two half equations Multiply the equations so that the number of electrons in each is the same Add the two equations and cancel out the electrons on either side If necessary, cancel any other species which appear on both sides Construct balanced redox equations for the reactions between. . . Mg and H+ Cr 2 O 72 - and Fe 2+ H 2 O 2 and Mn. O 4¯ C 2 O 42 - and Mn. O 4¯ S 2 O 32 - and I 2 Cr 2 O 72 - and I¯

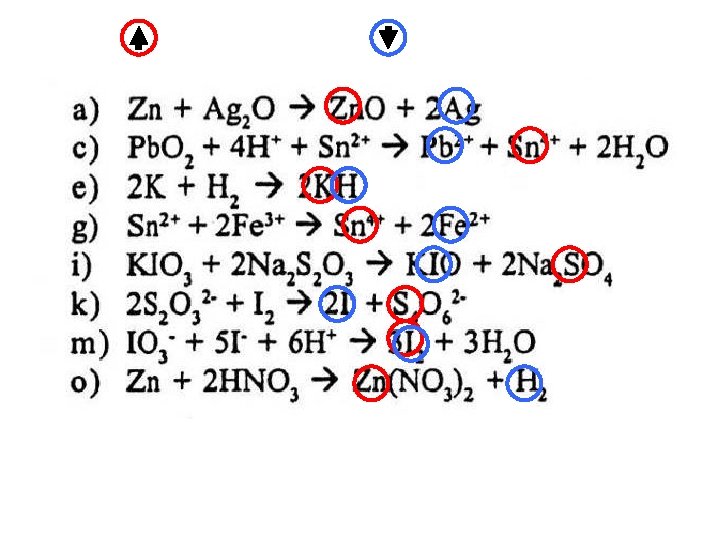
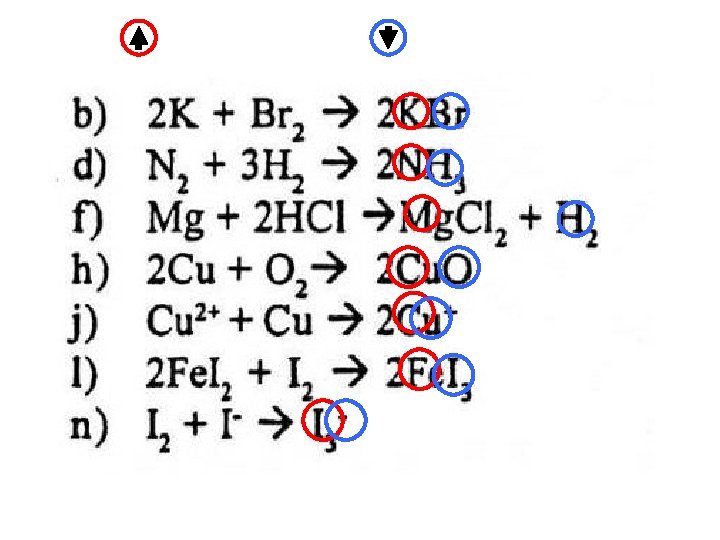
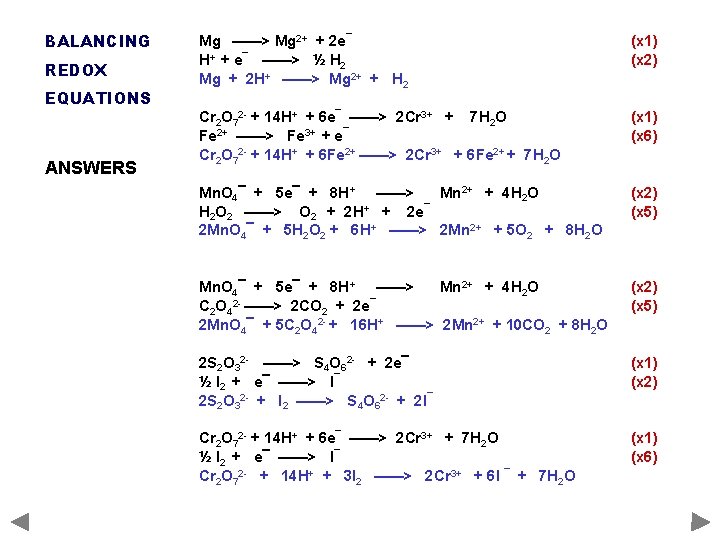
BALANCING REDOX EQUATIONS ANSWERS Mg ——> Mg 2+ + 2 e¯ H+ + e¯ ——> ½ H 2 Mg + 2 H+ ——> Mg 2+ + H 2 (x 1) (x 2) Cr 2 O 72 - + 14 H+ + 6 e¯ ——> 2 Cr 3+ + 7 H 2 O Fe 2+ ——> Fe 3+ + e¯ Cr 2 O 72 - + 14 H+ + 6 Fe 2+ ——> 2 Cr 3+ + 6 Fe 2+ + 7 H 2 O (x 1) (x 6) Mn. O 4¯ + 5 e¯ + 8 H+ ——> Mn 2+ + 4 H 2 O 2 ——> O 2 + 2 H+ + 2 e¯ 2 Mn. O 4¯ + 5 H 2 O 2 + 6 H+ ——> 2 Mn 2+ + 5 O 2 + 8 H 2 O (x 2) (x 5) Mn. O 4¯ + 5 e¯ + 8 H+ ——> Mn 2+ + 4 H 2 O C 2 O 42 - ——> 2 CO 2 + 2 e¯ 2 Mn. O 4¯ + 5 C 2 O 42 - + 16 H+ ——> 2 Mn 2+ + 10 CO 2 + 8 H 2 O (x 2) (x 5) 2 S 2 O 32 - ——> S 4 O 62 - + 2 e¯ ½ I 2 + e¯ ——> I¯ 2 S 2 O 32 - + I 2 ——> S 4 O 62 - + 2 I¯ (x 1) (x 2) Cr 2 O 72 - + 14 H+ + 6 e¯ ——> 2 Cr 3+ + 7 H 2 O ½ I 2 + e¯ ——> I¯ Cr 2 O 72 - + 14 H+ + 3 I 2 ——> 2 Cr 3+ + 6 I ¯ + 7 H 2 O (x 1) (x 6)


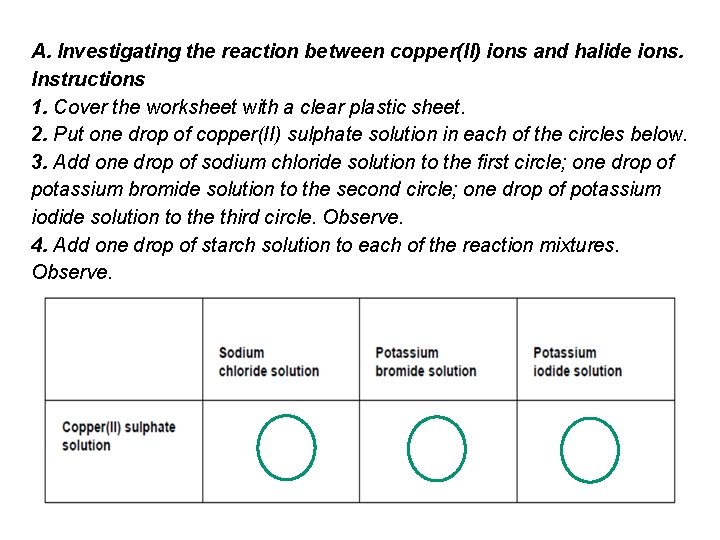
A. Investigating the reaction between copper(II) ions and halide ions. Instructions 1. Cover the worksheet with a clear plastic sheet. 2. Put one drop of copper(II) sulphate solution in each of the circles below. 3. Add one drop of sodium chloride solution to the first circle; one drop of potassium bromide solution to the second circle; one drop of potassium iodide solution to the third circle. Observe. 4. Add one drop of starch solution to each of the reaction mixtures. Observe.

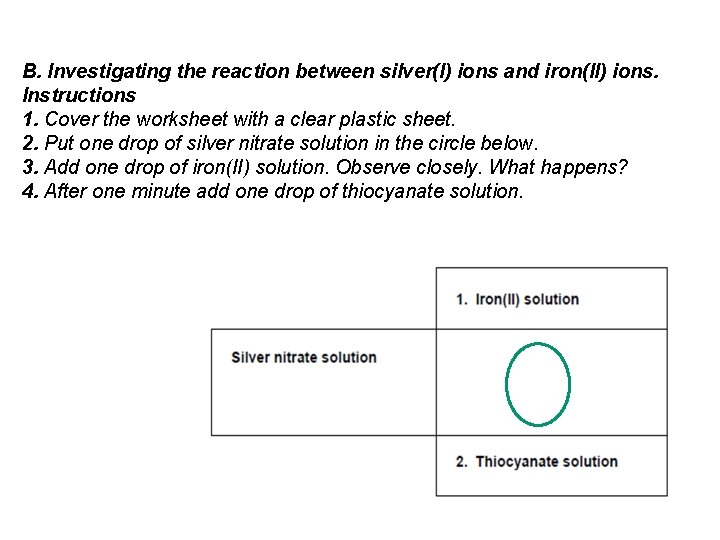
B. Investigating the reaction between silver(I) ions and iron(II) ions. Instructions 1. Cover the worksheet with a clear plastic sheet. 2. Put one drop of silver nitrate solution in the circle below. 3. Add one drop of iron(II) solution. Observe closely. What happens? 4. After one minute add one drop of thiocyanate solution.

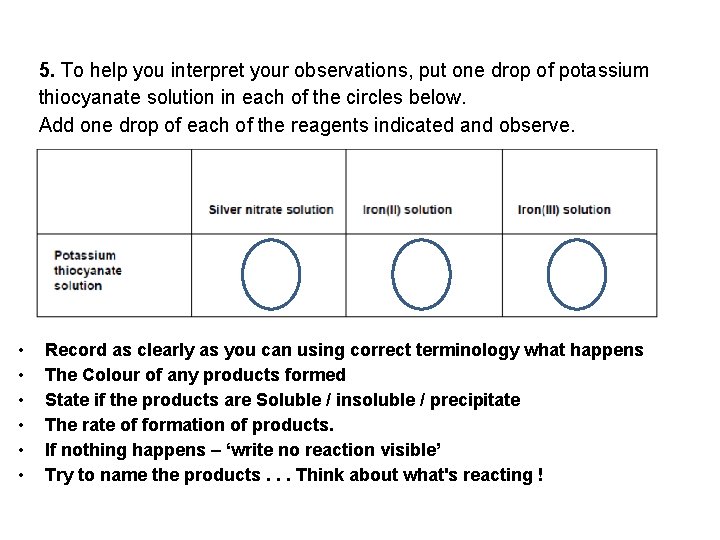
5. To help you interpret your observations, put one drop of potassium thiocyanate solution in each of the circles below. Add one drop of each of the reagents indicated and observe. • • • Record as clearly as you can using correct terminology what happens The Colour of any products formed State if the products are Soluble / insoluble / precipitate The rate of formation of products. If nothing happens – ‘write no reaction visible’ Try to name the products. . . Think about what's reacting !

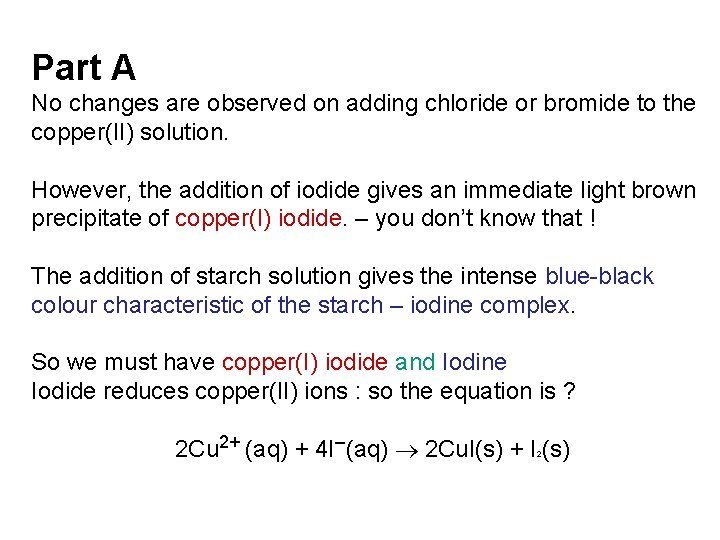
Part A No changes are observed on adding chloride or bromide to the copper(II) solution. However, the addition of iodide gives an immediate light brown precipitate of copper(I) iodide. – you don’t know that ! The addition of starch solution gives the intense blue-black colour characteristic of the starch – iodine complex. So we must have copper(I) iodide and Iodine Iodide reduces copper(II) ions : so the equation is ? 2 Cu 2+ (aq) + 4 I–(aq) 2 Cu. I(s) + I (s) 2

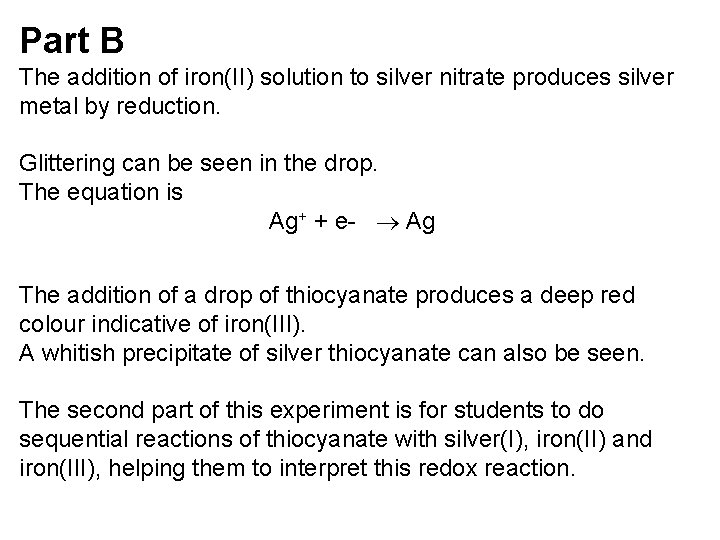
Part B The addition of iron(II) solution to silver nitrate produces silver metal by reduction. Glittering can be seen in the drop. The equation is Ag+ + e- Ag The addition of a drop of thiocyanate produces a deep red colour indicative of iron(III). A whitish precipitate of silver thiocyanate can also be seen. The second part of this experiment is for students to do sequential reactions of thiocyanate with silver(I), iron(II) and iron(III), helping them to interpret this redox reaction.

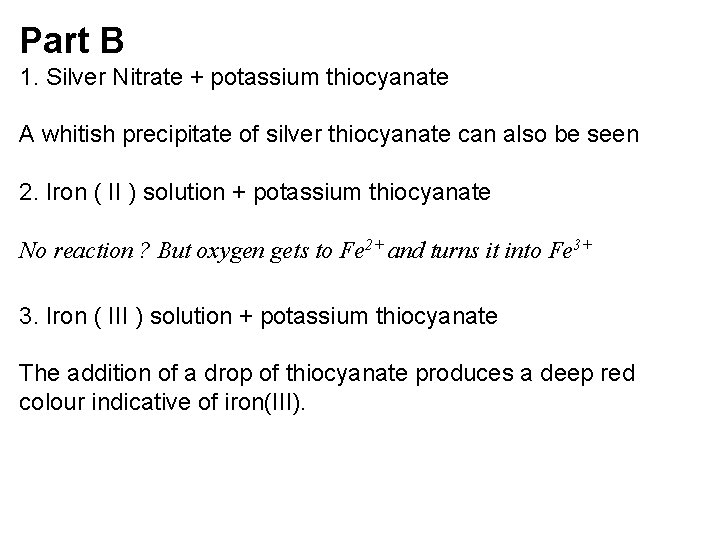
Part B 1. Silver Nitrate + potassium thiocyanate A whitish precipitate of silver thiocyanate can also be seen 2. Iron ( II ) solution + potassium thiocyanate No reaction ? But oxygen gets to Fe 2+ and turns it into Fe 3+ 3. Iron ( III ) solution + potassium thiocyanate The addition of a drop of thiocyanate produces a deep red colour indicative of iron(III).

Ele Negative men oxidation t states − 4 hydr oge n heli um lithi um bery lliu m boro n carb − 4 on nitro gen − 3 phos phor us sulf ur chlo rine argo n pota ssiu m calc ium scan diu m titan ium van adiu m chro miu m man gan ese − 1 0 +1 − 1 H +1 − 3 − 2 − 1 +4 B +1 +2 +3 C +1 +5 +6 +7 +8 +9 [2] +2 +3 +4 − 1 N +1 +2 +3 +4 − 1 O +1 +2 − 1 F − 1 Na +1 Mg +5 Ne [3] +1 +2 Al +1 +2 +3 − 2 − 1 Si +1 +2 +3 +4 − 3 − 2 − 1 P +1 +2 +3 +4 − 2 [4] +5 − 1 S +1 +2 +3 +4 +5 +6 − 1 Cl +1 +2 +3 +4 +5 +6 +7 Ar − 1 − 3 +1 [5] +2 Sc +1 +2 +3 Ti +1 +2 +3 +4 V +1 +2 +3 +4 +5 − 2 − 1 Cr +1 +2 +3 +4 +5 +6 − 2 − 1 Mn +1 +2 +3 +4 +5 +6 +6 − 1 Fe +1 +2 − 1 Co +1 +2 − 1 Ni +1 − 2 − 1 − 3 − 2 − 1 − 2 − 3 +3 +4 +5 +2 +3 +4 Cu +1 +2 +3 +4 +1 +2 Ga +1 +2 +3 Ge +1 +3 +1 +2 Br +1 +2 +5 [11] +7 Rb +1 Sr +1 +2 Y +1 +2 [12] [13] [14] +3 +1 +2 +3 +4 +5 Mo +1 +2 +3 +4 +5 +6 − 1 Tc +1 +2 +3 +4 +5 +6 +7 Ru +1 +2 +3 +4 +5 +6 +7 Rh +1 +2 +3 +4 +5 +6 Pd +1 +2 Ag +1 +2 Cd +1 +2 In +1 +2 − 1 +4 +3 Xe +1 Cs +1 [18] [19] +4 +2 +1 [16] [17] +6 +4 +3 I [15] +8 +3 +2 Te − 1 +3 +2 +5 +4 Ba +2 La +2 +3 Ce +2 +3 +4 Pr +2 +3 +4 Nd +2 +3 +4 Pm +2 +3 Sm +2 +3 Eu +2 +3 Gd +1 +2 +3 Tb +1 +6 [20] +7 +6 [22] [23] +2 +3 +4 [24] +2 +3 +4 [25] Ho +2 +3 Er +2 +3 Tm +2 +3 Yb +2 +3 [26] [27] +3 Hf +2 +3 +4 − 1 Ta +2 +3 +4 +5 − 1 W +1 +2 +3 +4 +5 +6 − 1 Re +1 +2 +3 +4 +5 +6 +7 − 1 Os +1 +2 +3 +4 +5 +6 +7 +8 − 1 Ir +1 +2 +3 +4 +5 +6 +7 +8 − 1 Pt +1 +2 +3 +4 +5 +6 +3 − 1 lead − 4 Au +1 +2 Hg +1 +2 Tl +1 Pb − 3 Bi − 2 At Fr [36] +3 +1 [37] +5 +4 +3 +5 [38] +6 +5 +2 +7 [39] [40] [41] +6 +1 Ra +2 Ac +2 +3 Th [35] +4 +2 Rn +1 [42] +2 +3 +4 Pa +2 +3 +4 U +2 [43] [44] +5 [45] +3 +4 +5 +6 Np +3 +4 +5 +6 +7 Pu +3 +4 +5 +6 +7 Am +2 +3 +4 Cm +2 +3 +4 Bk +2 +3 +4 Cf +2 +3 +4 Es +2 +3 +4 Fm +2 +3 Md +2 +3 No +2 +3 Lr Rf Db Sg Bh Hs [29] [30] [31] [32] [33] [34] +3 +1 [28] +9 +5 +4 +2 Po − 1 [21] +8 Dy Lu gold +4 +6 Zr − 2 mer cury thall ium +3 [10] +5 +4 +2 Sb − 2 [9] +4 Nb − 3 − 2 [8] +2 As Se Sn − 2 [7] − 1 − 3 +7 − 1 − 2 − 3 [6] Zn Kr neo dym ium pro met hiu m sam ariu m euro piu m gad olini um terbi um dysp rosi um hol miu m erbi um thuli um ytter biu m lutet ium hafn ium tant alu m tung sten rhen ium osmi um iridi um plati num +1 Ca − 1 − 4 anti mon y tellu rium iodi ne xen on caes ium bari um lant han um ceri um pras eod ymi um K − 1 − 2 galli um ger man − 4 ium arse nic sele niu m bro min e kryp ton rubi diu m stro ntiu m yttri um zirc oniu m niob ium mol ybd enu m tech neti um ruth eniu m rhod ium pall adiu m silve r cad miu m indi um ruth erfo rdiu m dub niu m seab orgi um bohr ium hass ium +2 − 2 zinc lawr enci um +1 +1 − 3 iron bism uth polo niu m astat ine rado n fran ciu m radi um acti niu m thori um prot acti niu m uran ium nept uniu m plut oniu m ame riciu m curi um berk eliu m calif orni um einst eini um fer miu m men dele viu m nob eliu m Notes +3 Li Be − 2 cob alt nick el cop per tin +2 He − 3 oxy gen fluo rine neo n sodi um mag nesi um alu mini um silic − 4 on Positive oxidation states − 2 +6 +8 [46] [47] +8 [48] [49] [50] [51] [52] +3 +4 [53] +5 [54] +6 [55] +7 +8 [56]