REDOX Oxidation and Reduction Reactions Chapters 20 and















- Slides: 41

REDOX!!! Oxidation and Reduction Reactions Chapters 20 and 21

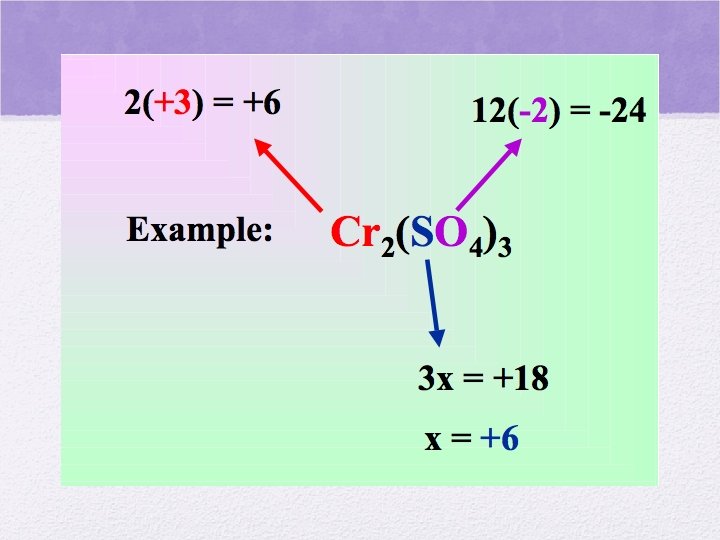
Oxidation Numbers • The electrical charge that an atom or ion has- or appears to have- when combined with other elements in a compound or polyatomic ion

Assigning Oxidation Numbers • Free elements= 0 charge • Group 1 metals= +1 • Li+, Na+, K+ • Group 2 metals= +2 • Ca 2+, Mg 2+ • Halogens= -1 • F-, Cl-

More oxidation numbers • Oxygen: always -2 • except peroxides (such as H 2 O 2) where each O is -1 • OR in combination with fluorine, OF 2, where O is +2 • Hydrogen: always +1 • except metal hydrides (where it is -1) • Li. H, Ca. H 2

And more rules • The total of the oxidation numbers in neutral compounds must equal 0 • The oxidation numbers of all the atoms in an ion, (including polyatomics) must add up to the charge on the ion


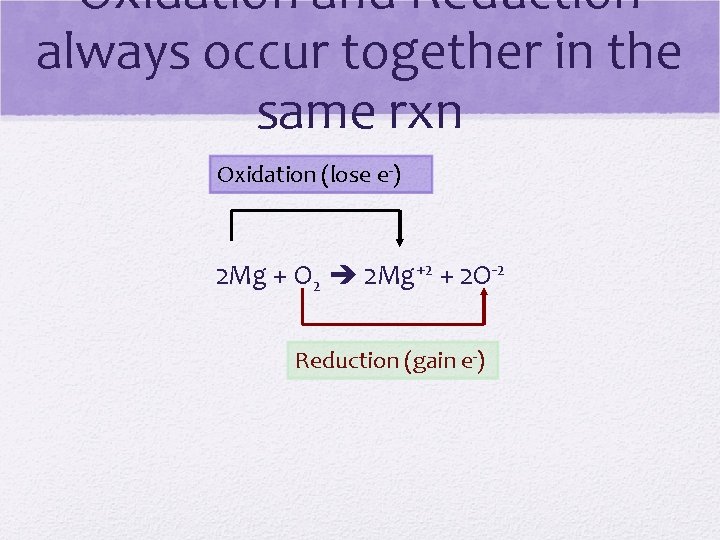
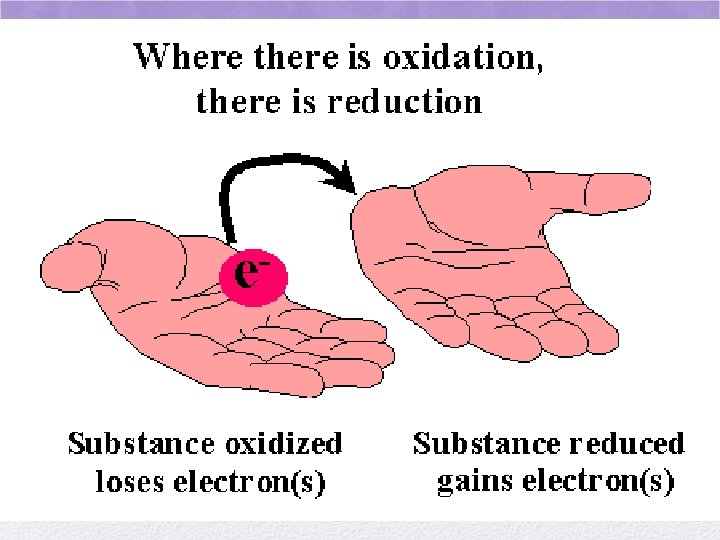
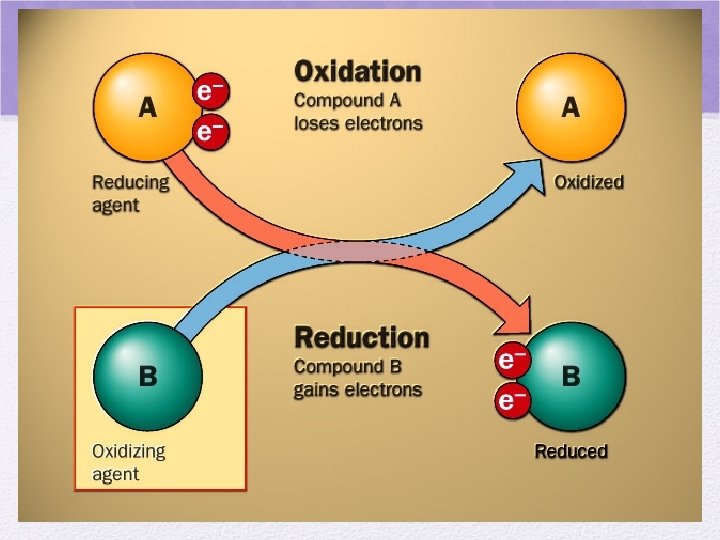
A REDOX rxn involves the transfer of electrons • REDUCTION: gain electrons • GER • Oxidation number is reduced • OXIDATION: lose electrons • LEO • Oxidation number gets bigger

LIGER

Oxidation and Reduction always occur together in the same rxn Oxidation (lose e-) 2 Mg + O 2 2 Mg+2 + 2 O-2 Reduction (gain e-)


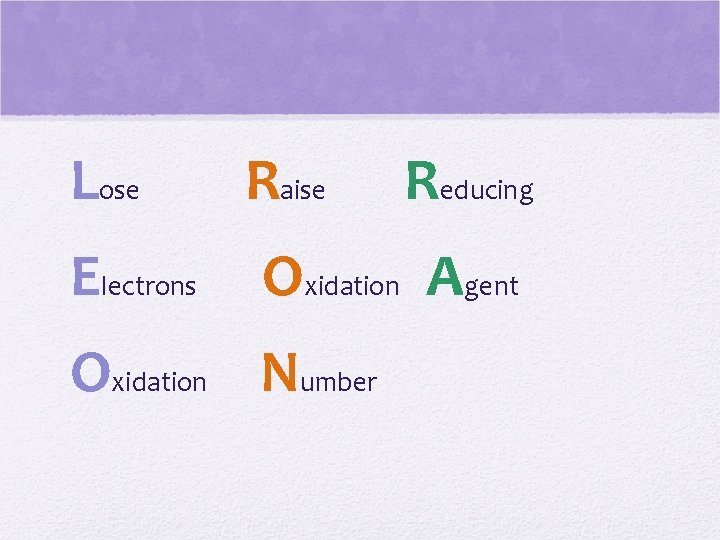
More on oxidation… • An element loses electrons • becomes oxidized • Charge becomes MORE POSITIVE • This particle reduces another by letting it “take” its electrons • reducing agent • Group 1: STRONG reducing agents • strong tendency to lose electrons

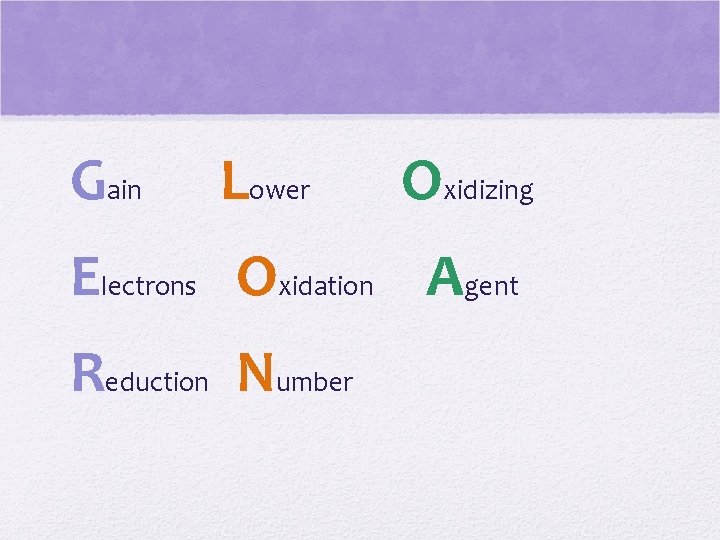
Lose Raise Electrons Oxidation Agent Oxidation Number Reducing

Reduction • An element gains electrons • Becomes reduced- lower oxidation # • Oxidation number decreases • Becomes more negative • This particle oxidizes another particle by removing an e- from it • Oxidizing agent • Group 17: STRONG OXIDIZING AGENTS • Strong tendency to accept e- and become reduced

Gain Lower Oxidizing Electrons Oxidation Agent Reduction Number


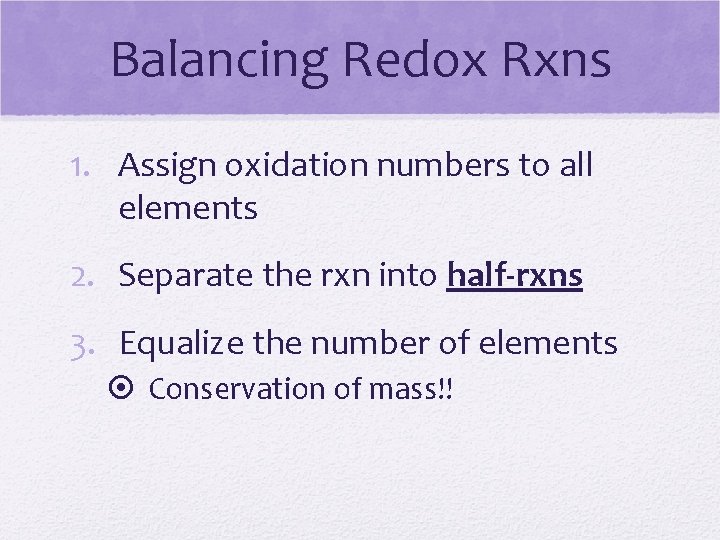
Balancing Redox Rxns 1. Assign oxidation numbers to all elements 2. Separate the rxn into half-rxns 3. Equalize the number of elements Conservation of mass!!

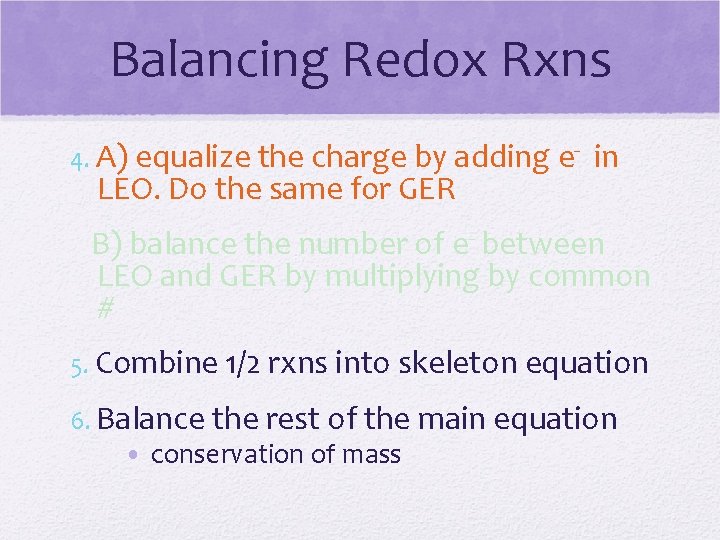
Balancing Redox Rxns 4. A) equalize the charge by adding e- LEO. Do the same for GER in B) balance the number of e- between LEO and GER by multiplying by common # 5. Combine 1/2 rxns into skeleton equation 6. Balance the rest of the main equation • conservation of mass

Types of Reactions • Single replacement, decomposition, synthesis: ALWAYS redox • Double replacement: NOT redox

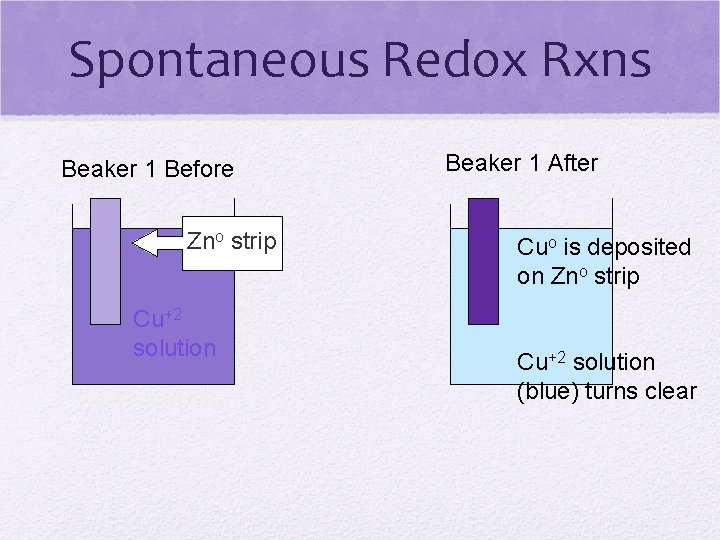
Spontaneous Redox Rxns • For any TWO METALS in an activity series (TABLE J), the more active metal is the more readily oxidized • Metals want to lose electrons • Halogens: the more active is more easily reduced • due to higher electronegativity • Nonmetals want to gain electrons

Spontaneous Redox Rxns Beaker 1 Before Zno strip Cu+2 solution Beaker 1 After Cuo is deposited on Zno strip Cu+2 solution (blue) turns clear

What is happening? • Atom (Zno) comes before the ion (Cu+2) on Table J • SPONTANEOUS • Metals WANT to lose electrons, so more active metal is oxidized • Ion is “forced” to gain electrons and become reduced

Table J: A Summary • Used to determine the direction of a spontaneous reaction • Recall: if one reactant is reduced, the other MUST BE OXIDIZED

METALS • Strong tendency to lose electrons • Undergo oxidation (LEO) • Arranged from best oxidizers at the top • Element higher up starts as the atom (0 charge) and gets oxidized (becomes + ion)

NONMETALS • Strong tendency to gain electrons (undergo reduction become - ) • Arranged from best reducers at the top

Electrochemistry • The study of the conversion of chemical energy to electrical energy • Electrochemical cell- converts chemical energy into electrical energy or electrical energy into chemical energy

Redox Rxns: electrons move from the oxidized element to the reduced • LEOxidation ----> GEReduction • IDEA: put the flow of electrons through a wire! • Electricity!!!

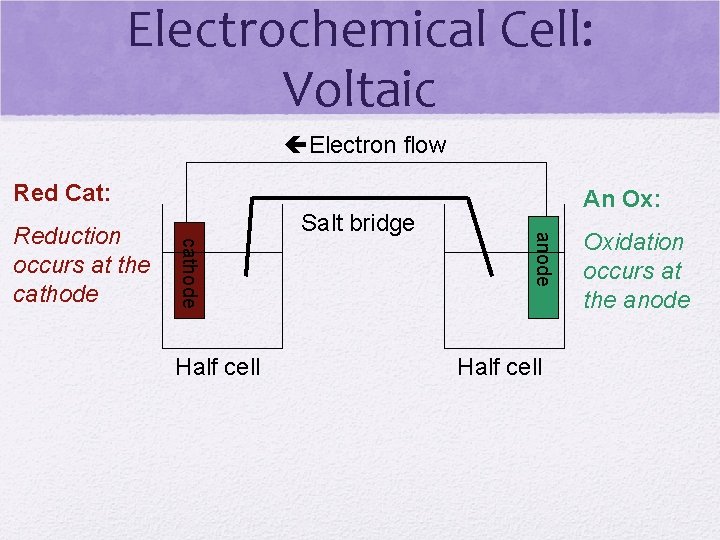
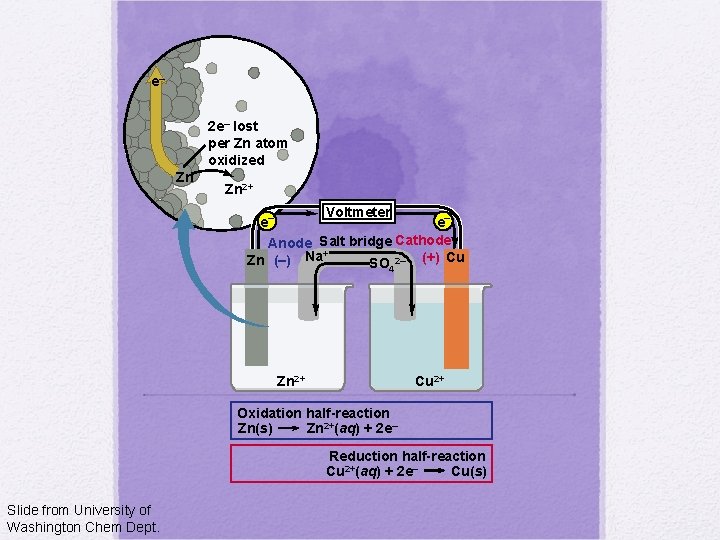
Electrochemical Cell: Voltaic Electron flow Red Cat: Half cell anode cathode Reduction occurs at the cathode Salt bridge An Ox: Half cell Oxidation occurs at the anode

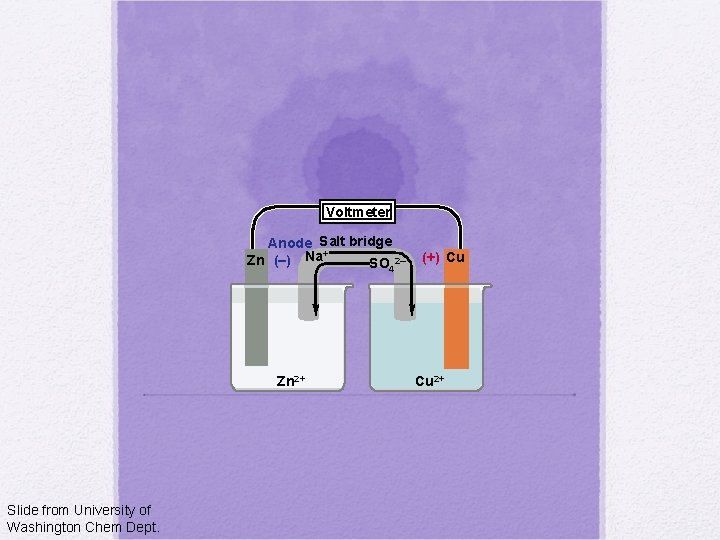
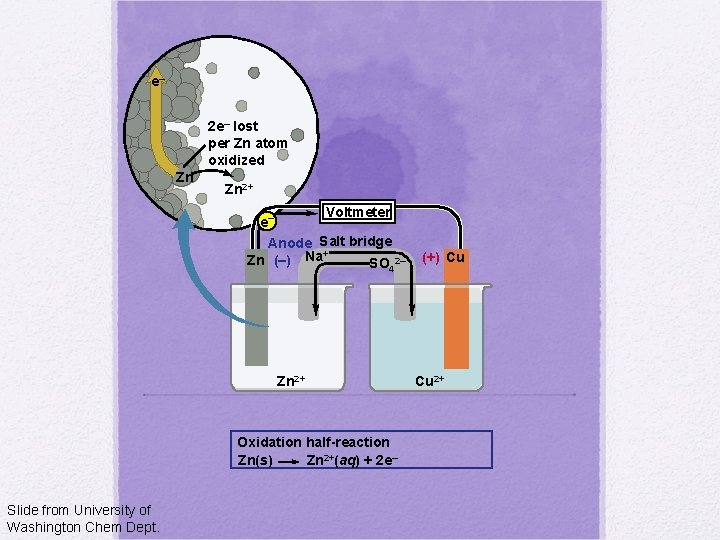
Voltmeter Anode Salt bridge + Zn (–) Na SO 2– 4 Zn 2+ Slide from University of Washington Chem Dept. (+) Cu Cu 2+

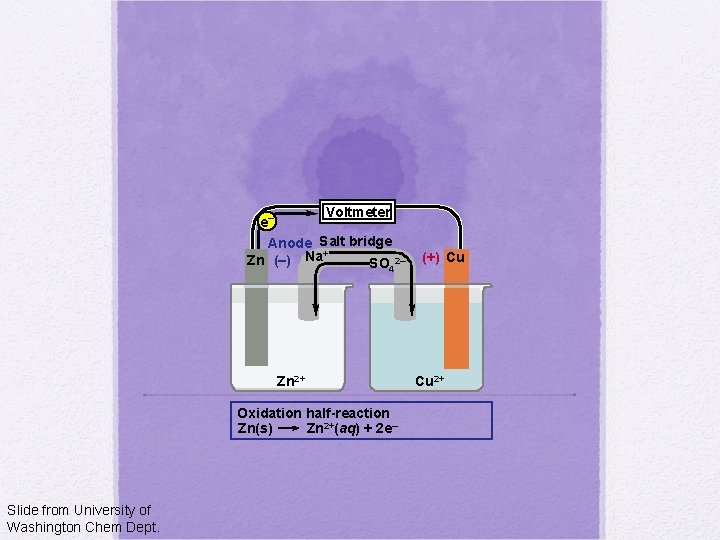
Voltmeter e– Anode Salt bridge + Zn (–) Na SO 2– 4 Zn 2+ Oxidation half-reaction Zn(s) Zn 2+(aq) + 2 e– Slide from University of Washington Chem Dept. (+) Cu Cu 2+

e– 2 e– lost per Zn atom oxidized Zn Zn 2+ Voltmeter e– Anode Salt bridge + Zn (–) Na SO 2– 4 Zn 2+ Oxidation half-reaction Zn(s) Zn 2+(aq) + 2 e– Slide from University of Washington Chem Dept. (+) Cu Cu 2+

e– 2 e– lost per Zn atom oxidized Zn Zn 2+ Voltmeter e– e– Anode Salt bridge Cathode + Zn (–) Na SO 2– (+) Cu 4 Zn 2+ Cu 2+ Oxidation half-reaction Zn(s) Zn 2+(aq) + 2 e– Reduction half-reaction Cu 2+(aq) + 2 e– Cu(s) Slide from University of Washington Chem Dept.

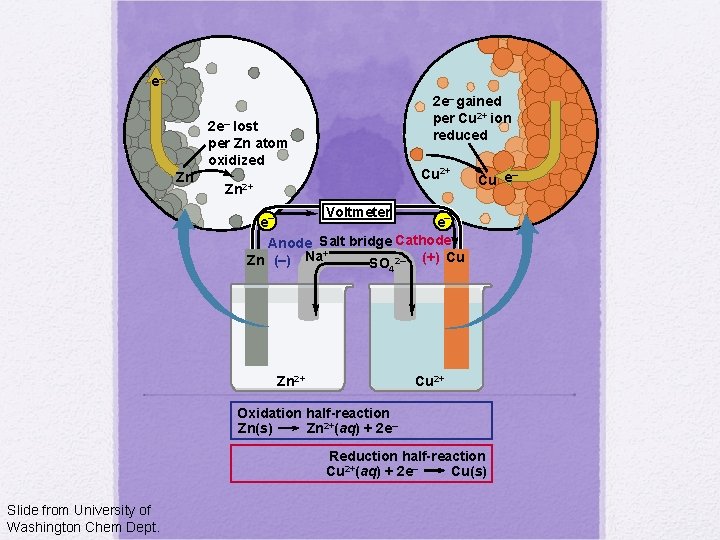
e– 2 e– gained per Cu 2+ ion reduced 2 e– lost per Zn atom oxidized Zn Cu 2+ Zn 2+ – Cu e Voltmeter e– e– Anode Salt bridge Cathode + Zn (–) Na SO 2– (+) Cu 4 Zn 2+ Cu 2+ Oxidation half-reaction Zn(s) Zn 2+(aq) + 2 e– Reduction half-reaction Cu 2+(aq) + 2 e– Cu(s) Slide from University of Washington Chem Dept.

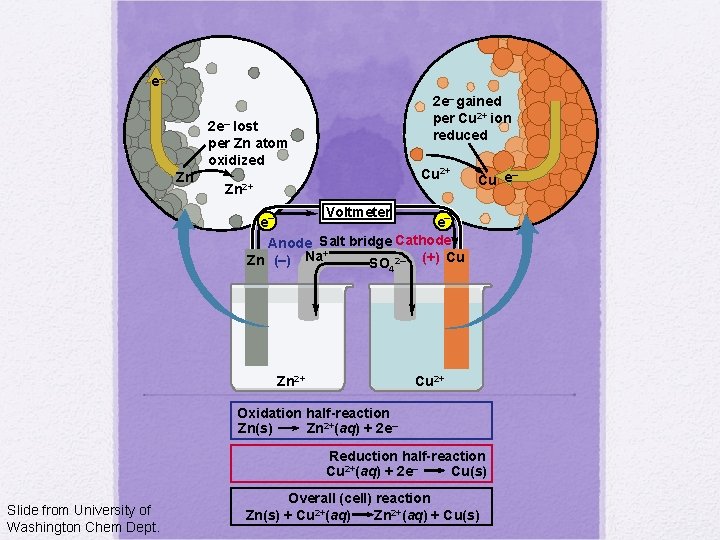
e– 2 e– gained per Cu 2+ ion reduced 2 e– lost per Zn atom oxidized Zn Cu 2+ Zn 2+ – Cu e Voltmeter e– e– Anode Salt bridge Cathode + Zn (–) Na SO 2– (+) Cu 4 Zn 2+ Cu 2+ Oxidation half-reaction Zn(s) Zn 2+(aq) + 2 e– Reduction half-reaction Cu 2+(aq) + 2 e– Cu(s) Slide from University of Washington Chem Dept. Overall (cell) reaction Zn 2+(aq) + Cu(s) Zn(s) + Cu 2+(aq)

Voltaic Cell • Salt Bridge: when e- move one solution will become very negative, e- won’t want to go there. • The salt bridge allows for ION MIGRATION • Without a salt bridge, the circuit is incomplete and e- cannot flow through the wire.

Electrolysis • The process by which electrical energy is used to make nonspontaneous redox rxns proceed

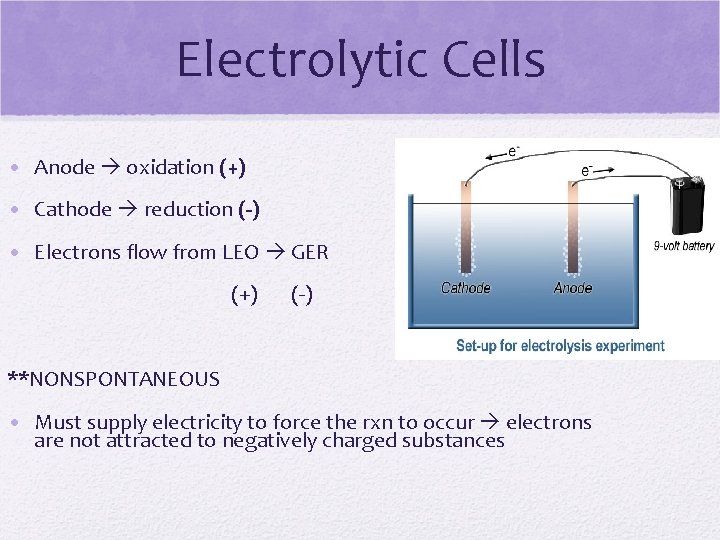
Electrolytic Cells • Anode oxidation (+) • Cathode reduction (-) • Electrons flow from LEO GER (+) (-) **NONSPONTANEOUS • Must supply electricity to force the rxn to occur electrons are not attracted to negatively charged substances

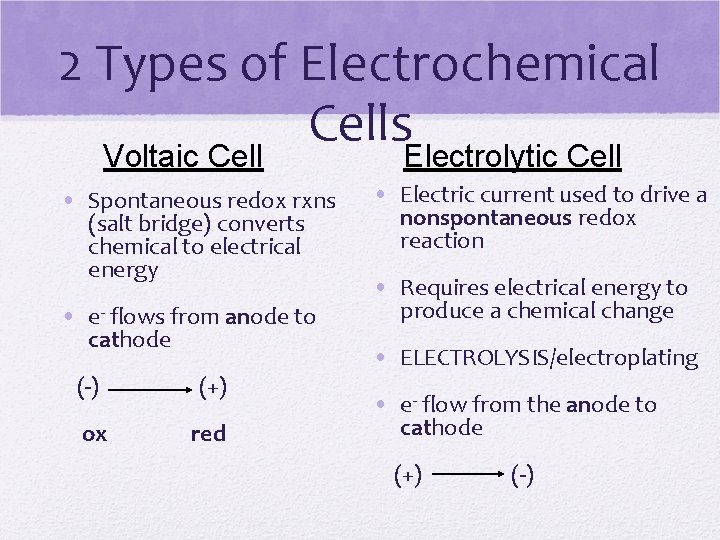
2 Types of Electrochemical Cells Voltaic Cell Electrolytic Cell • Spontaneous redox rxns (salt bridge) converts chemical to electrical energy • e- flows from anode to cathode (-) (+) ox red • Electric current used to drive a nonspontaneous redox reaction • Requires electrical energy to produce a chemical change • ELECTROLYSIS/electroplating • e- flow from the anode to cathode (+) (-)

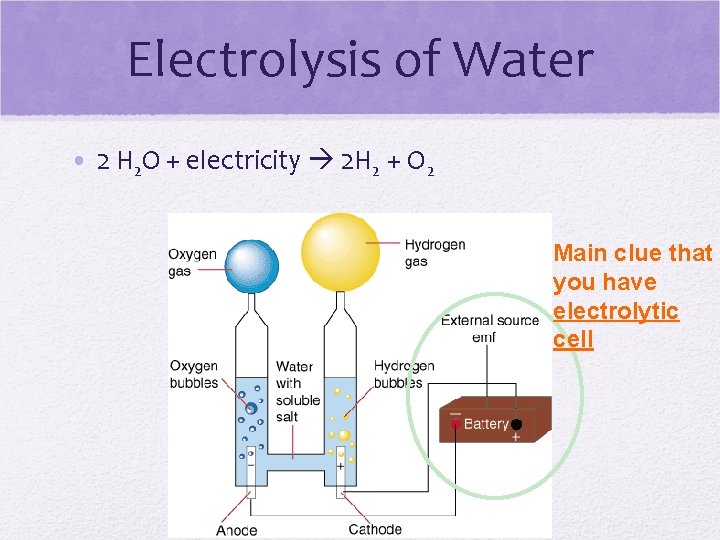
Electrolysis of Water • 2 H 2 O + electricity 2 H 2 + O 2 Main clue that you have electrolytic cell

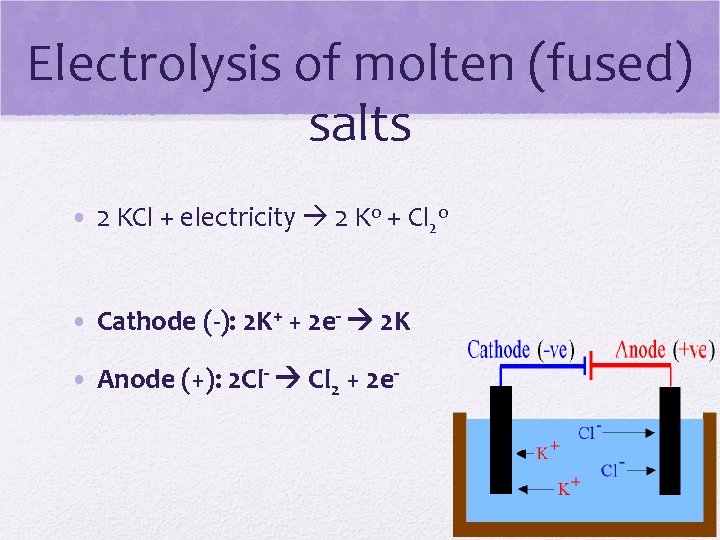
Electrolysis of molten (fused) salts • 2 KCl + electricity 2 Ko + Cl 2 o • Cathode (-): 2 K+ + 2 e- 2 K • Anode (+): 2 Cl- Cl 2 + 2 e-

Electroplating • Silver, chrome, stainless steel plating • Electric current is used (electrolytic) • Nonspontaneous reaction • Result: cover a surface with metal plating (spoon, car bumper, etc. )

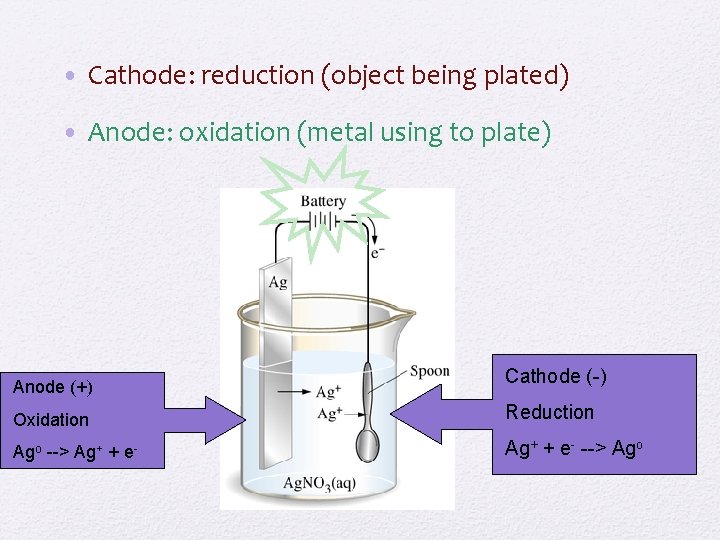
• Cathode: reduction (object being plated) • Anode: oxidation (metal using to plate) Anode (+) Cathode (-) Oxidation Reduction Ago --> Ag+ + e- --> Ago