Redox Geochemistry WHY Redox gradients drive life processes



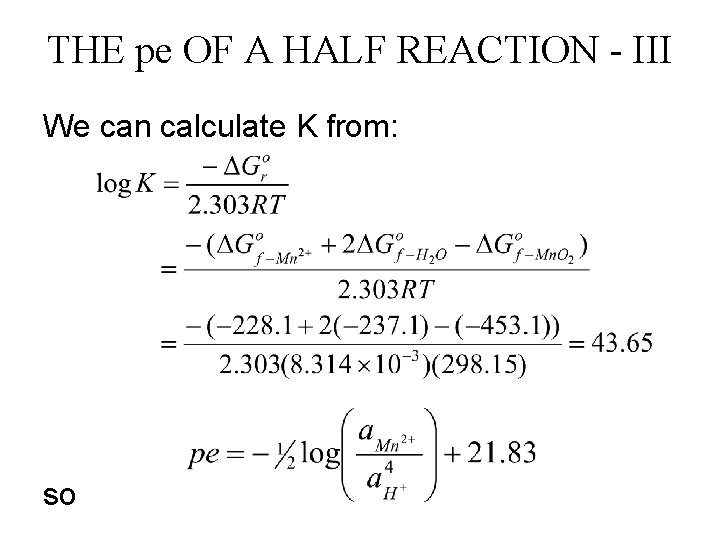




- Slides: 39

Redox Geochemistry

WHY? • Redox gradients drive life processes! – The transfer of electrons between oxidants and reactants is harnessed as the battery, the source of metabolic energy for organisms • Metal mobility redox state of metals and ligands that may complex them is the critical factor in the solubility of many metals – Contaminant transport – Ore deposit formation

REDOX CLASSIFICATION OF NATURAL WATERS Oxic waters - waters that contain measurable dissolved oxygen. Suboxic waters - waters that lack measurable oxygen or sulfide, but do contain significant dissolved iron (> ~0. 1 mg L-1). Reducing waters (anoxic) - waters that contain both dissolved iron and sulfide.

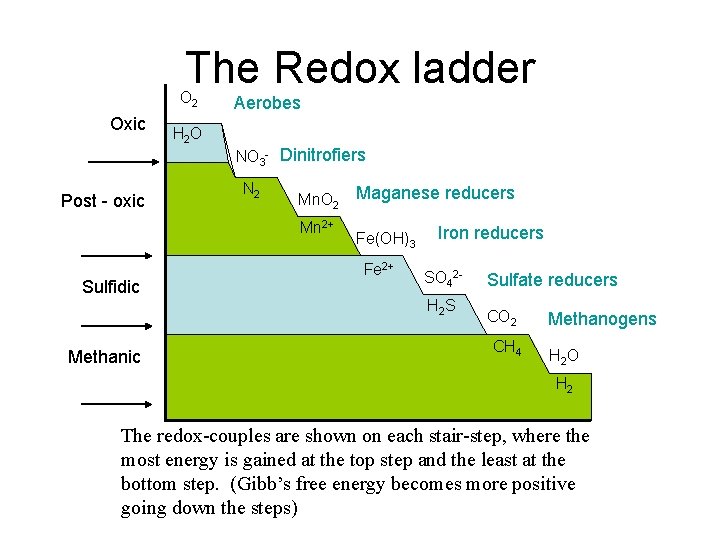
The Redox ladder O 2 Oxic Post - oxic H 2 O Aerobes NO 3 - Dinitrofiers N 2 Mn. O 2 Mn 2+ Sulfidic Methanic Maganese reducers Fe(OH)3 Fe 2+ Iron reducers SO 42 H 2 S Sulfate reducers CO 2 CH 4 Methanogens H 2 O H 2 The redox-couples are shown on each stair-step, where the most energy is gained at the top step and the least at the bottom step. (Gibb’s free energy becomes more positive going down the steps)

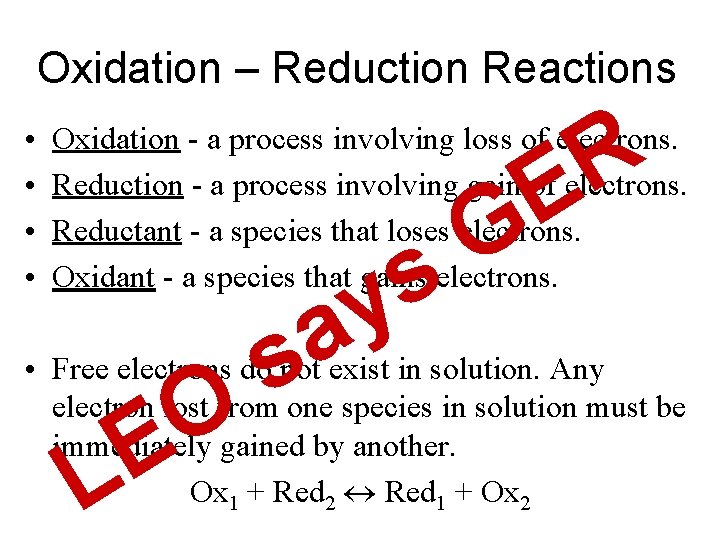
Oxidation – Reduction Reactions • • R E Oxidation - a process involving loss of electrons. Reduction - a process involving gain of electrons. Reductant - a species that loses electrons. Oxidant - a species that gains electrons. G s y a s • Free electrons do not exist in solution. Any electron lost from one species in solution must be immediately gained by another. Ox 1 + Red 2 Red 1 + Ox 2 L O E

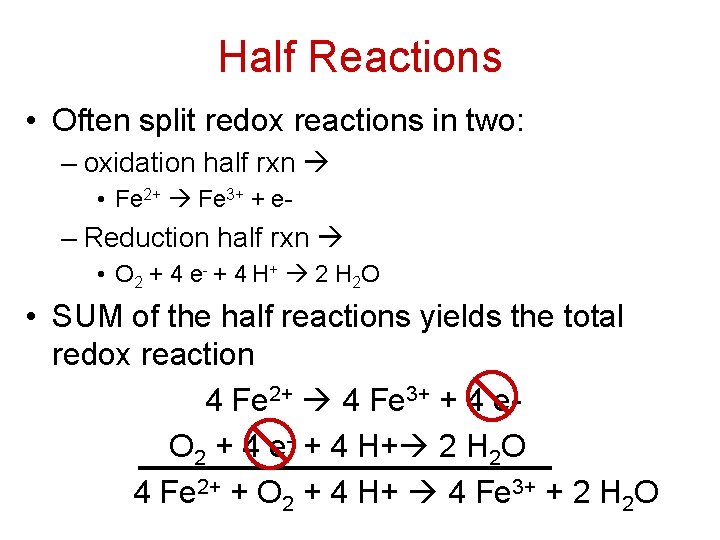
Half Reactions • Often split redox reactions in two: – oxidation half rxn • Fe 2+ Fe 3+ + e- – Reduction half rxn • O 2 + 4 e - + 4 H + 2 H 2 O • SUM of the half reactions yields the total redox reaction 4 Fe 2+ 4 Fe 3+ + 4 e. O 2 + 4 e- + 4 H+ 2 H 2 O 4 Fe 2+ + O 2 + 4 H+ 4 Fe 3+ + 2 H 2 O

Redox Couples • For any half reaction, the oxidized/reduced pair is the redox couple: – Fe 2+ Fe 3+ + e– Couple: Fe 2+/Fe 3+ – H 2 S + 4 H 2 O SO 42 - + 10 H+ + 8 e– Couple: H 2 S/SO 42 -

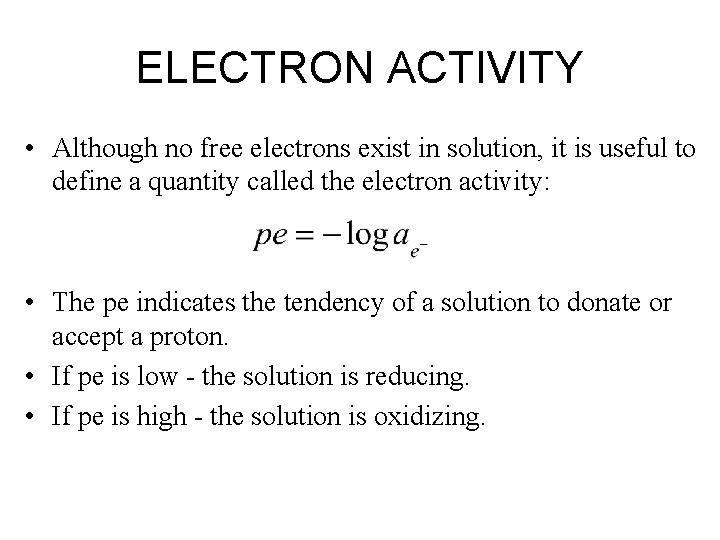
ELECTRON ACTIVITY • Although no free electrons exist in solution, it is useful to define a quantity called the electron activity: • The pe indicates the tendency of a solution to donate or accept a proton. • If pe is low - the solution is reducing. • If pe is high - the solution is oxidizing.

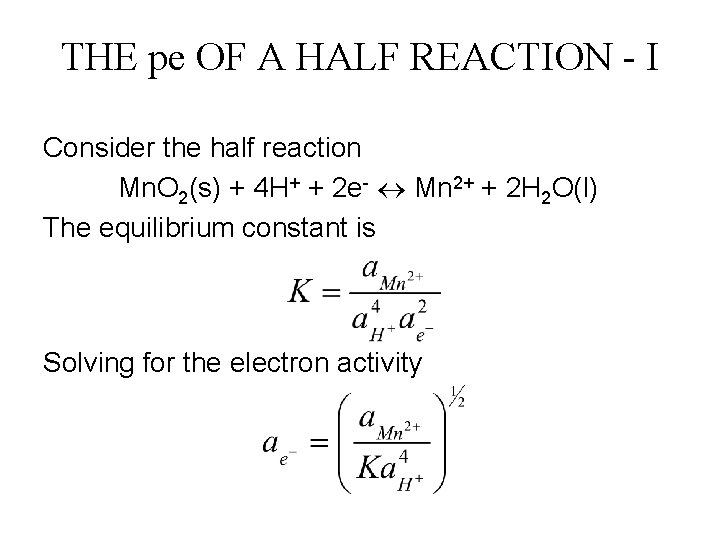
THE pe OF A HALF REACTION - I Consider the half reaction Mn. O 2(s) + 4 H+ + 2 e- Mn 2+ + 2 H 2 O(l) The equilibrium constant is Solving for the electron activity

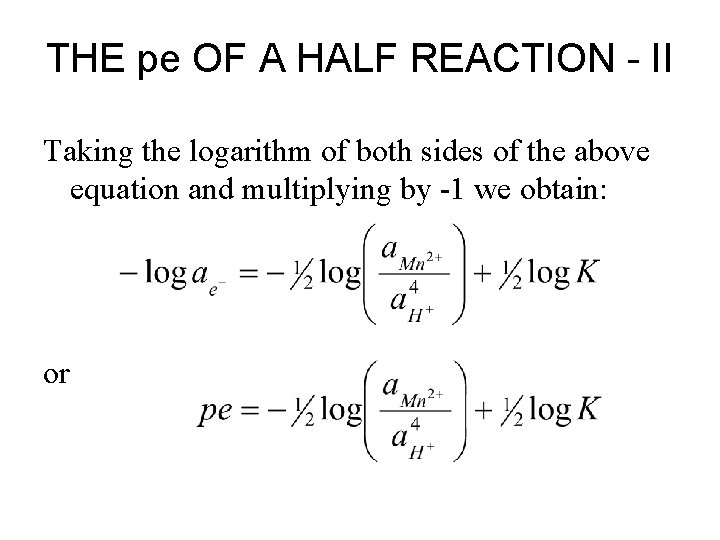
THE pe OF A HALF REACTION - II Taking the logarithm of both sides of the above equation and multiplying by -1 we obtain: or
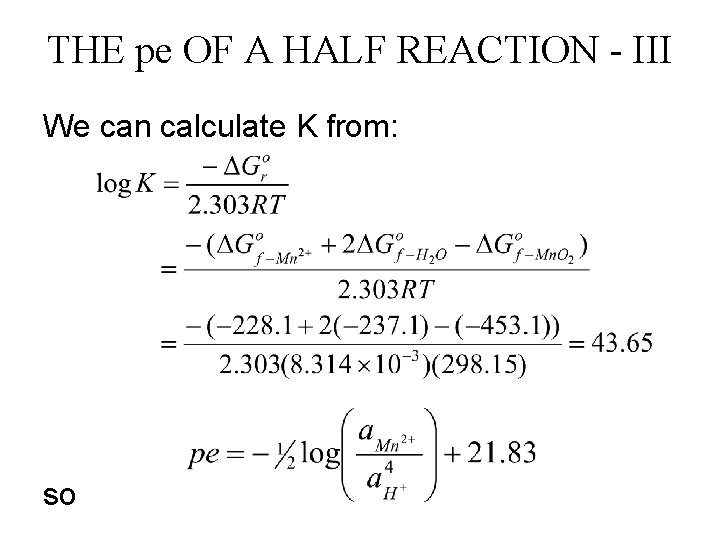
THE pe OF A HALF REACTION - III We can calculate K from: so

WE NEED A REFERENCE POINT! Values of pe are meaningless without a point of reference with which to compare. Such a point is provided by the following reaction: ½H 2(g) H+ + e. By convention so K = 1.

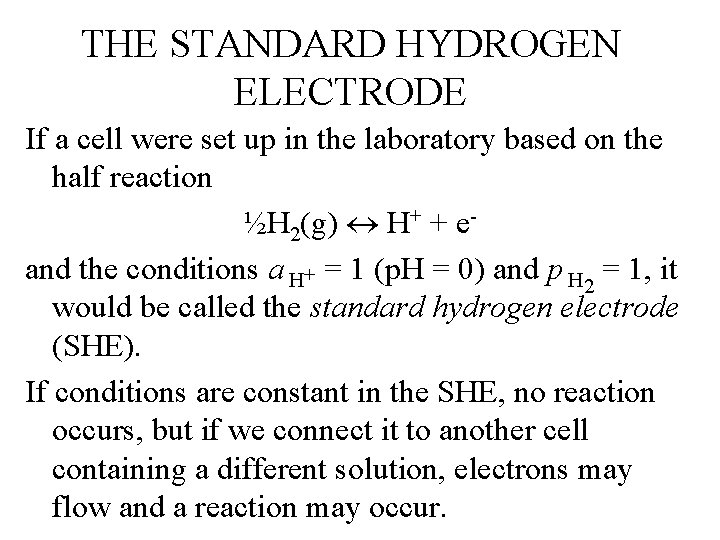
THE STANDARD HYDROGEN ELECTRODE If a cell were set up in the laboratory based on the half reaction ½H 2(g) H+ + eand the conditions a H+ = 1 (p. H = 0) and p H 2 = 1, it would be called the standard hydrogen electrode (SHE). If conditions are constant in the SHE, no reaction occurs, but if we connect it to another cell containing a different solution, electrons may flow and a reaction may occur.

STANDARD HYDROGEN ELECTRODE ½H 2(g) H+ + e-

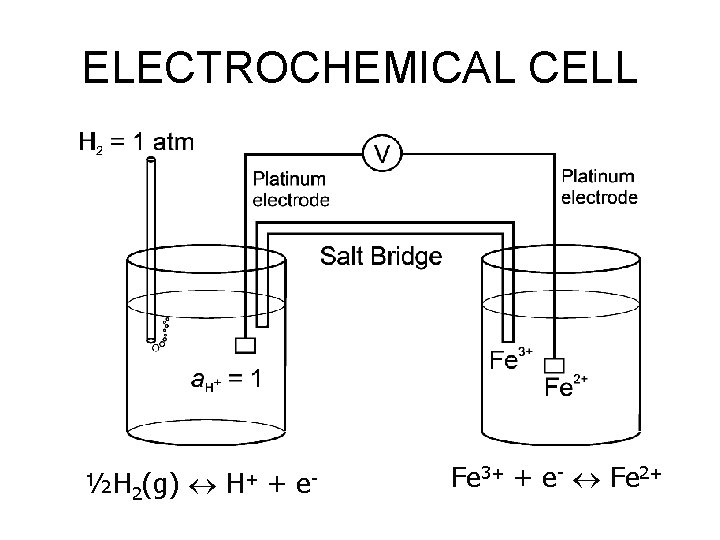
ELECTROCHEMICAL CELL ½H 2(g) H+ + e- Fe 3+ + e- Fe 2+

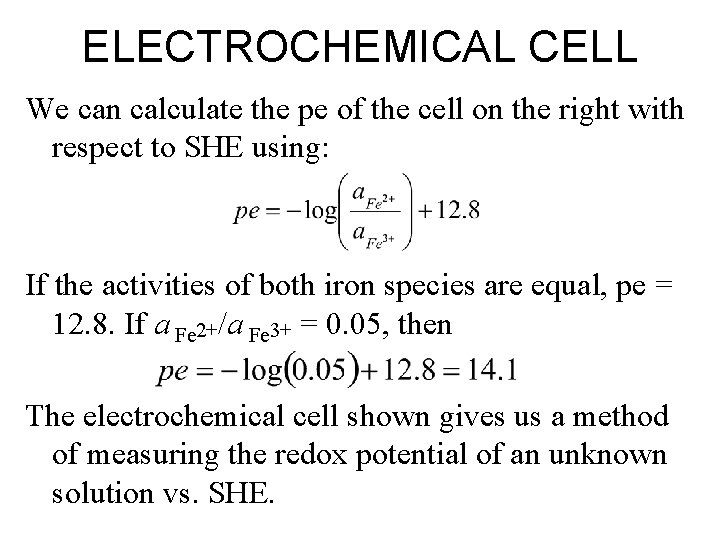
ELECTROCHEMICAL CELL We can calculate the pe of the cell on the right with respect to SHE using: If the activities of both iron species are equal, pe = 12. 8. If a Fe 2+/a Fe 3+ = 0. 05, then The electrochemical cell shown gives us a method of measuring the redox potential of an unknown solution vs. SHE.

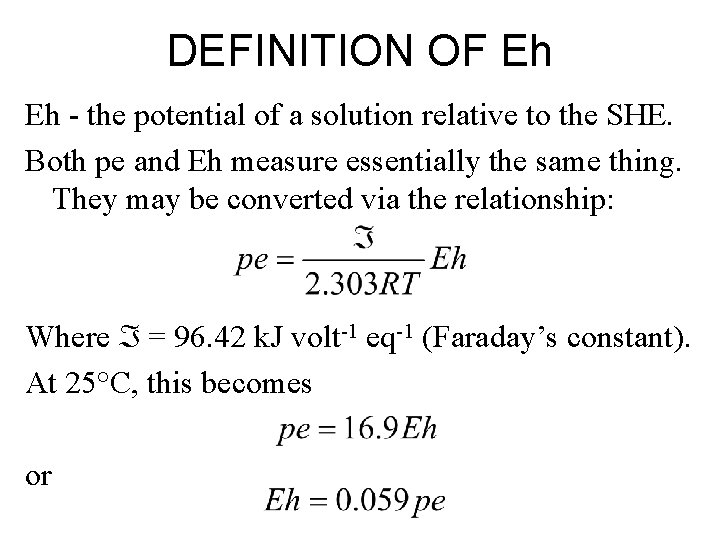
DEFINITION OF Eh Eh - the potential of a solution relative to the SHE. Both pe and Eh measure essentially the same thing. They may be converted via the relationship: Where = 96. 42 k. J volt-1 eq-1 (Faraday’s constant). At 25°C, this becomes or

Eh – Measurement and meaning • Eh is the driving force for a redox reaction • No exposed live wires in natural systems (usually…) where does Eh come from? • From Nernst redox couples exist at some Eh (Fe 2+/Fe 3+=1, Eh = +0. 77 V) • When two redox species (like Fe 2+ and O 2) come together, they should react towards equilibrium • Total Eh of a solution is measure of that equilibrium

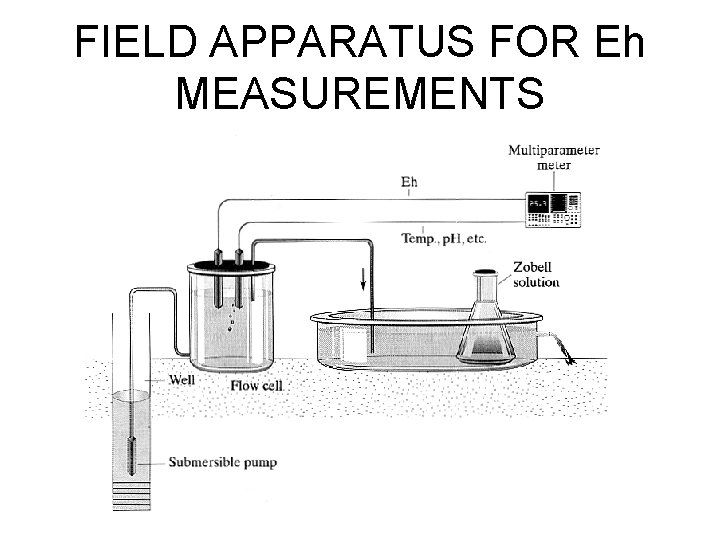
FIELD APPARATUS FOR Eh MEASUREMENTS

CALIBRATION OF ELECTRODES • The indicator electrode is usually platinum. • In practice, the SHE is not a convenient field reference electrode. • More convenient reference electrodes include saturated calomel (SCE - mercury in mercurous chloride solution) or silver-silver chloride electrodes. • A standard solution is employed to calibrate the electrode. • Zobell’s solution - solution of potassium ferric-ferro cyanide of known Eh.

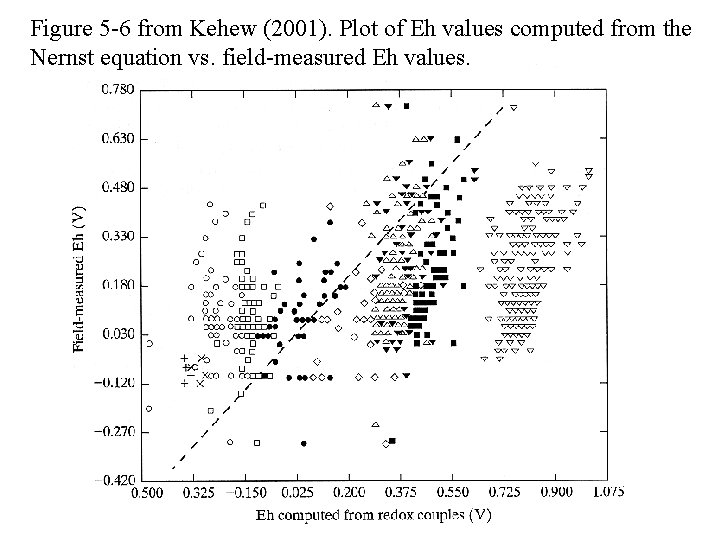
Figure 5 -6 from Kehew (2001). Plot of Eh values computed from the Nernst equation vs. field-measured Eh values.

PROBLEMS WITH Eh MEASUREMENTS • Natural waters contain many redox couples NOT at equilibrium; it is not always clear to which couple (if any) the Eh electrode is responding. • Eh values calculated from redox couples often do not correlate with each other or directly measured Eh values. • Eh can change during sampling and measurement if caution is not exercised. • Electrode material (Pt usually used, others also used) – Many species are not electroactive (do NOT react electrode) • Many species of O, N, C, As, Se, and S are not electroactive at Pt – electrode can become poisoned by sulfide, etc.

Other methods of determining the redox state of natural systems • For some, we can directly measure the redox couple (such as Fe 2+ and Fe 3+) • Techniques to directly measure redox SPECIES: – Amperometry (ion specific electrodes) – Voltammetry – Chromatography – Spectrophotometry/ colorimetry – EPR, NMR – Synchrotron based XANES, EXAFS, etc.

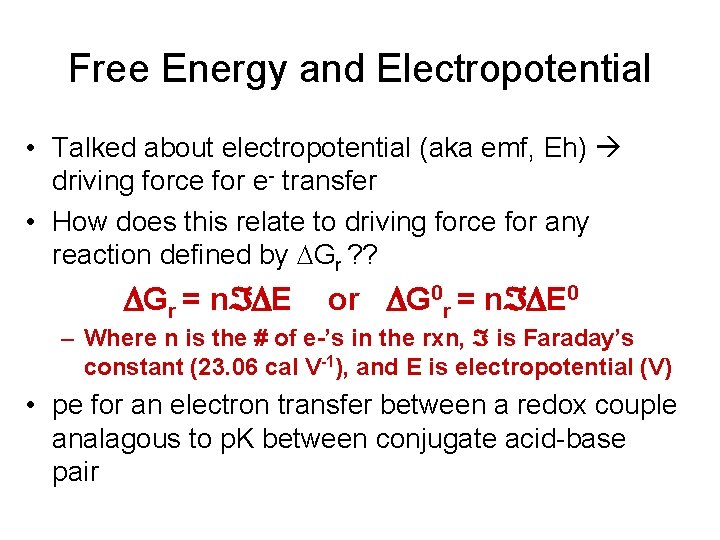
Free Energy and Electropotential • Talked about electropotential (aka emf, Eh) driving force for e- transfer • How does this relate to driving force for any reaction defined by Gr ? ? DGr = n DE or DG 0 r = n DE 0 – Where n is the # of e-’s in the rxn, is Faraday’s constant (23. 06 cal V-1), and E is electropotential (V) • pe for an electron transfer between a redox couple analagous to p. K between conjugate acid-base pair

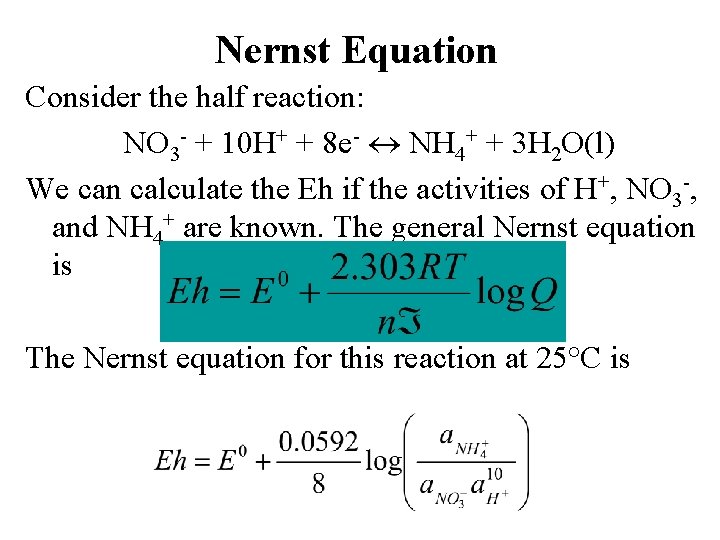
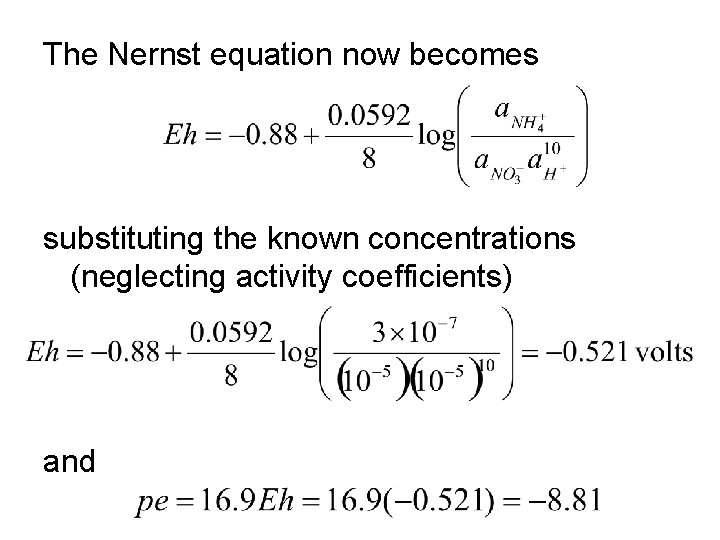
Nernst Equation Consider the half reaction: NO 3 - + 10 H+ + 8 e- NH 4+ + 3 H 2 O(l) We can calculate the Eh if the activities of H+, NO 3 -, and NH 4+ are known. The general Nernst equation is The Nernst equation for this reaction at 25°C is

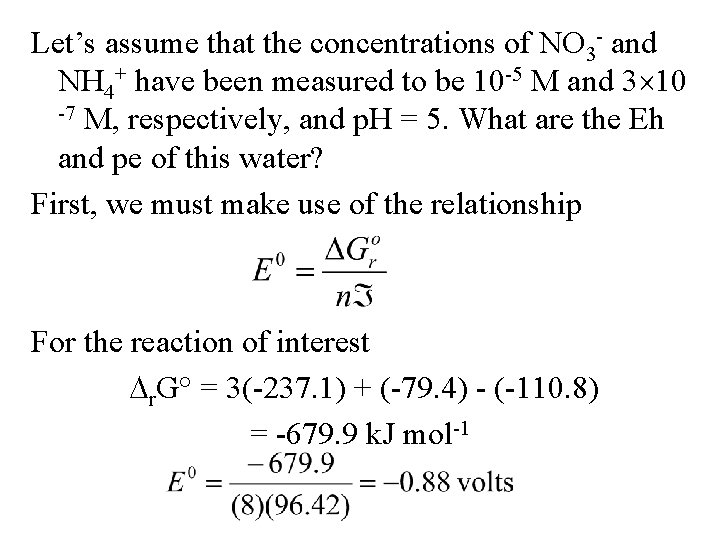
Let’s assume that the concentrations of NO 3 - and NH 4+ have been measured to be 10 -5 M and 3 10 -7 M, respectively, and p. H = 5. What are the Eh and pe of this water? First, we must make use of the relationship For the reaction of interest r. G° = 3(-237. 1) + (-79. 4) - (-110. 8) = -679. 9 k. J mol-1

The Nernst equation now becomes substituting the known concentrations (neglecting activity coefficients) and

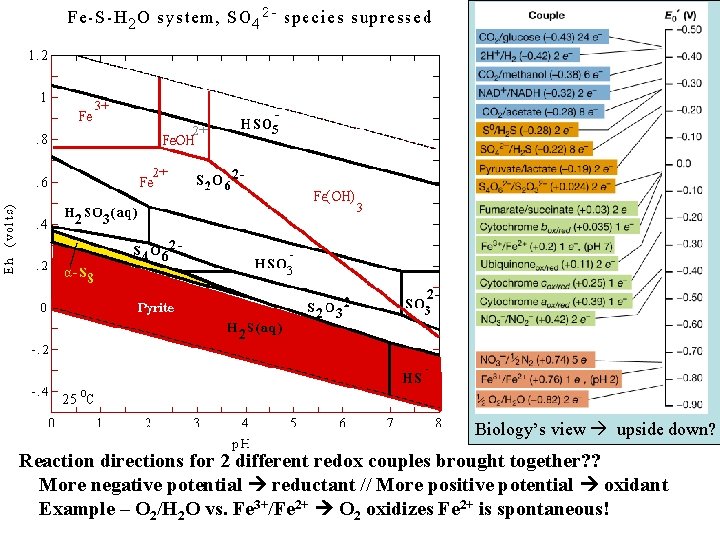
Biology’s view upside down? Reaction directions for 2 different redox couples brought together? ? More negative potential reductant // More positive potential oxidant Example – O 2/H 2 O vs. Fe 3+/Fe 2+ O 2 oxidizes Fe 2+ is spontaneous!

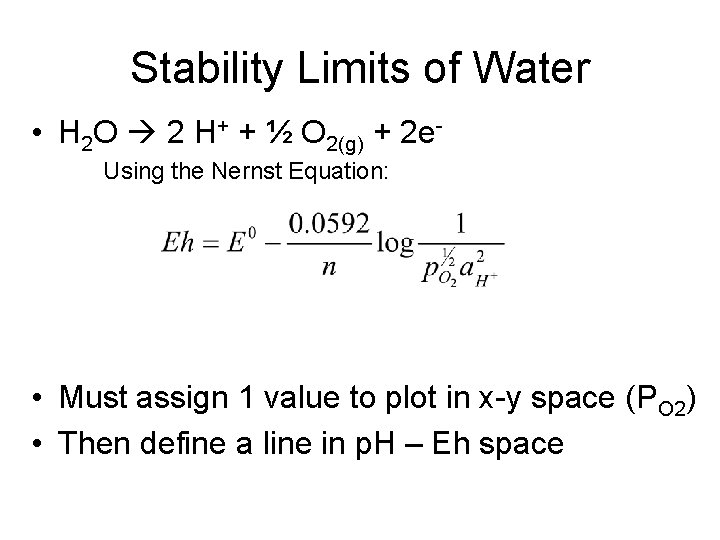
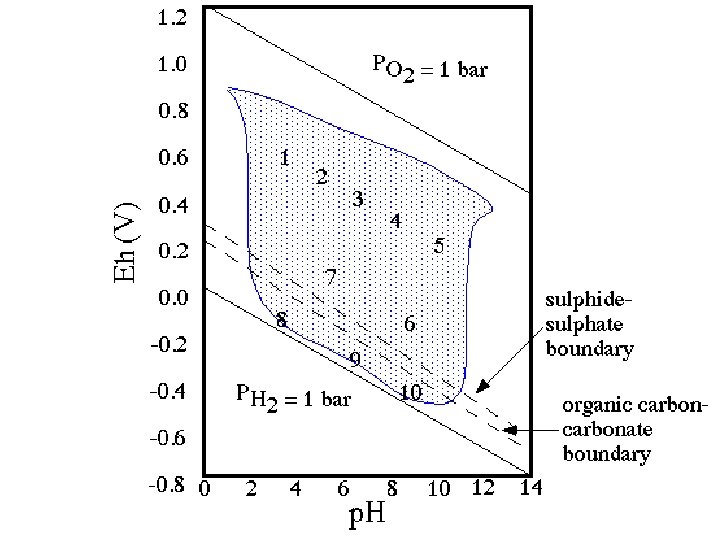
Stability Limits of Water • H 2 O 2 H+ + ½ O 2(g) + 2 e. Using the Nernst Equation: • Must assign 1 value to plot in x-y space (PO 2) • Then define a line in p. H – Eh space

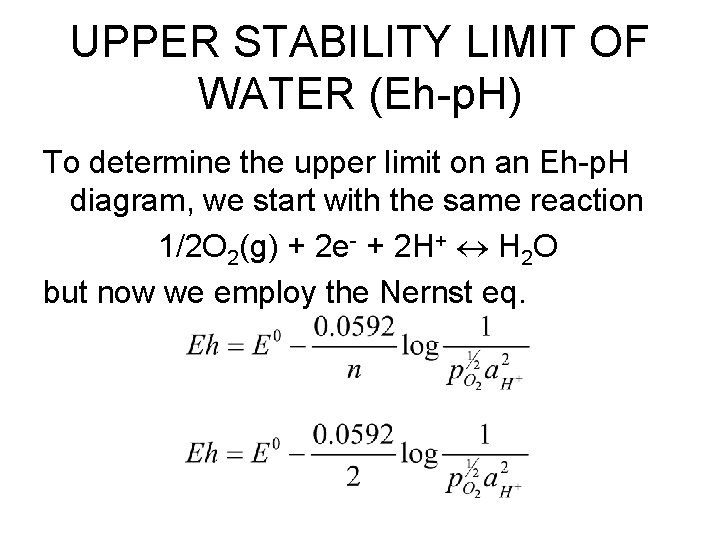
UPPER STABILITY LIMIT OF WATER (Eh-p. H) To determine the upper limit on an Eh-p. H diagram, we start with the same reaction 1/2 O 2(g) + 2 e- + 2 H+ H 2 O but now we employ the Nernst eq.

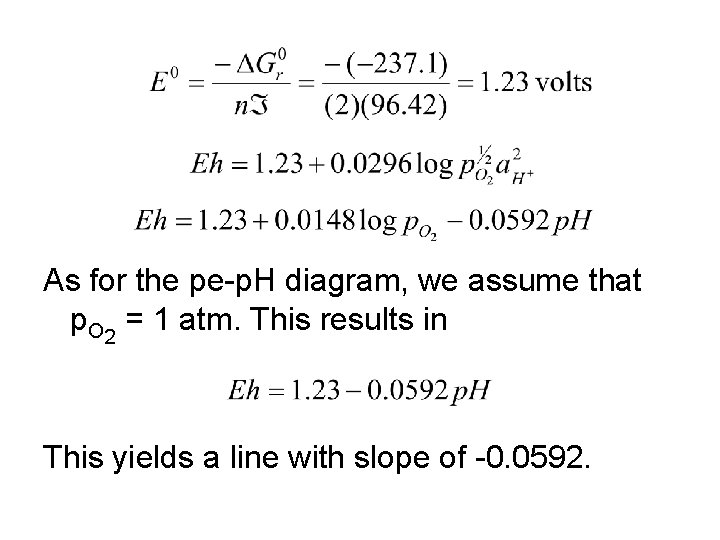
As for the pe-p. H diagram, we assume that p. O 2 = 1 atm. This results in This yields a line with slope of -0. 0592.

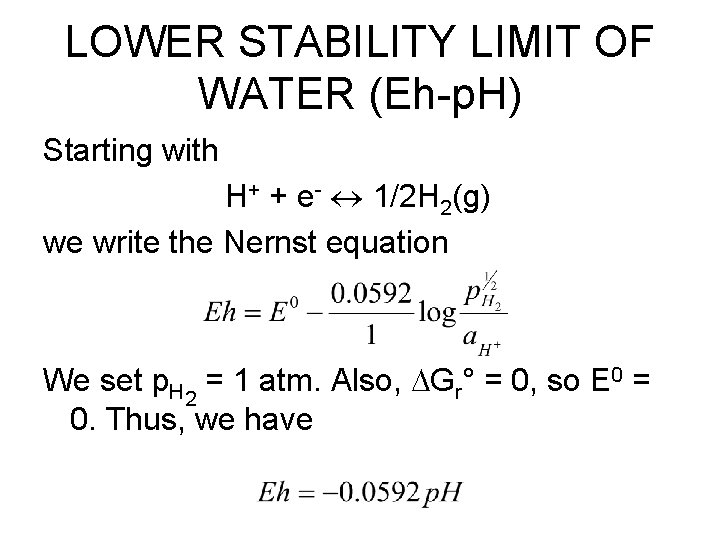
LOWER STABILITY LIMIT OF WATER (Eh-p. H) Starting with H+ + e- 1/2 H 2(g) we write the Nernst equation We set p. H 2 = 1 atm. Also, Gr° = 0, so E 0 = 0. Thus, we have


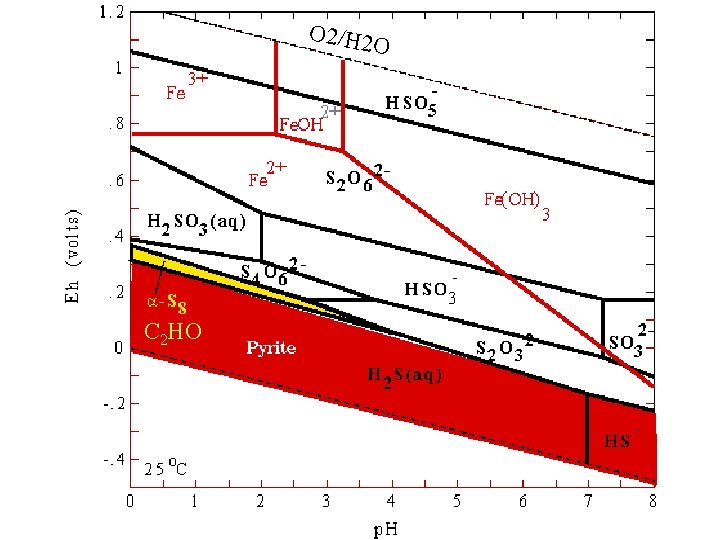
O 2/H 2 O C 2 HO

Making stability diagrams • For any reaction we wish to consider, we can write a mass action equation for that reaction • We make 2 -axis diagrams to represent how several reactions change with respect to 2 variables (the axes) • Common examples: Eh-p. H, PO 2 -p. H, T-[x], [x]-[y], [x]/[y]-[z], etc

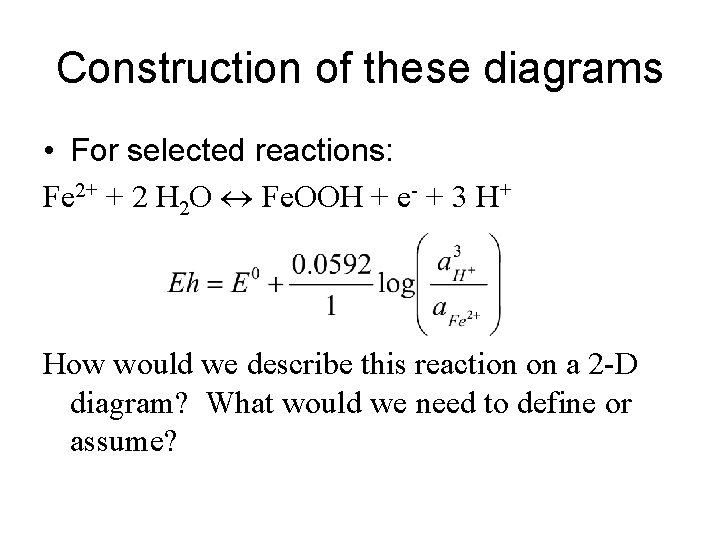
Construction of these diagrams • For selected reactions: Fe 2+ + 2 H 2 O Fe. OOH + e- + 3 H+ How would we describe this reaction on a 2 -D diagram? What would we need to define or assume?

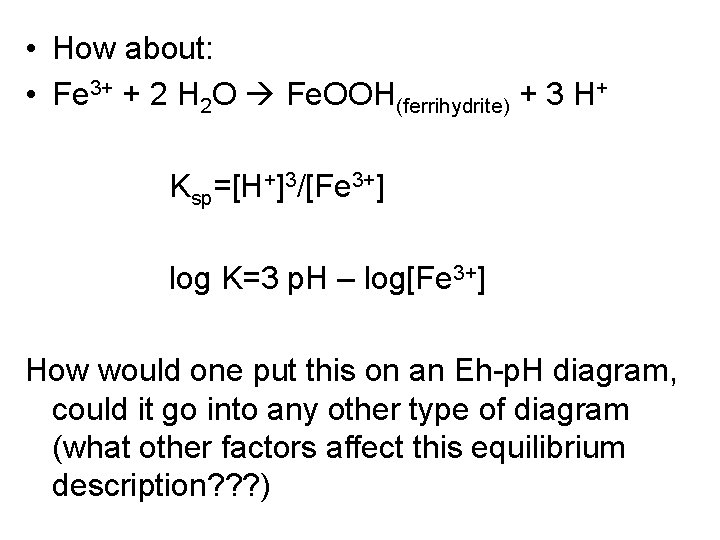
• How about: • Fe 3+ + 2 H 2 O Fe. OOH(ferrihydrite) + 3 H+ Ksp=[H+]3/[Fe 3+] log K=3 p. H – log[Fe 3+] How would one put this on an Eh-p. H diagram, could it go into any other type of diagram (what other factors affect this equilibrium description? ? ? )

Redox titrations • Imagine an oxic water being reduced to become an anoxic water • We can change the Eh of a solution by adding reductant or oxidant just like we can change p. H by adding an acid or base • Just as p. K determined which conjugate acid -base pair would buffer p. H, pe determines what redox pair will buffer Eh (and thus be reduced/oxidized themselves)

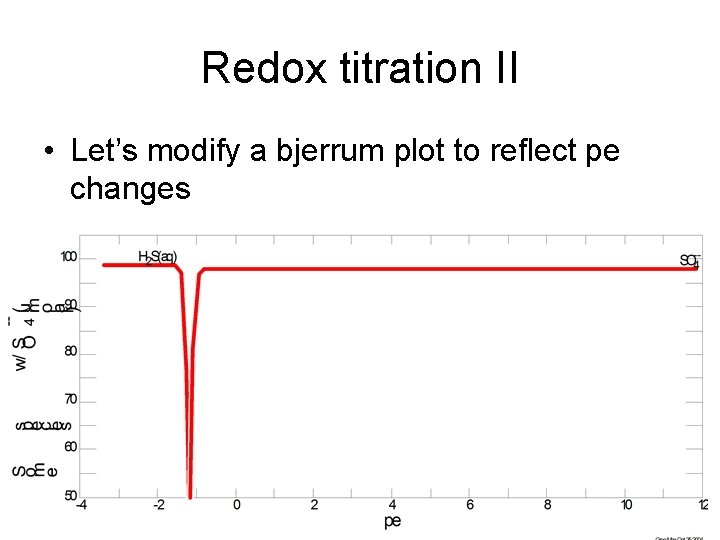
Redox titration II • Let’s modify a bjerrum plot to reflect pe changes