Redox Flow Batteries Regenerative Fuel Cells Enabling renewable

Redox Flow Batteries & Regenerative Fuel Cells Enabling renewable energy • • • Vanadium redox flow Polysulfide/Bromine flow Uranium (!!!) based Zinc/Bromide (half redox flow) All liquid regenerative fuel cells Ongoing Projects here in UIUC NPRE 498 Energy Storage
Redox flow battery (history) • Dated back to the 70’s with the 1973 oil crisis • Examples: – Fe(III)/Fe(II) in liquid (solvated ionic) form – Cr(III)/Cr(II) in liquid (solvated ionic) form NPRE 498 Energy Storage
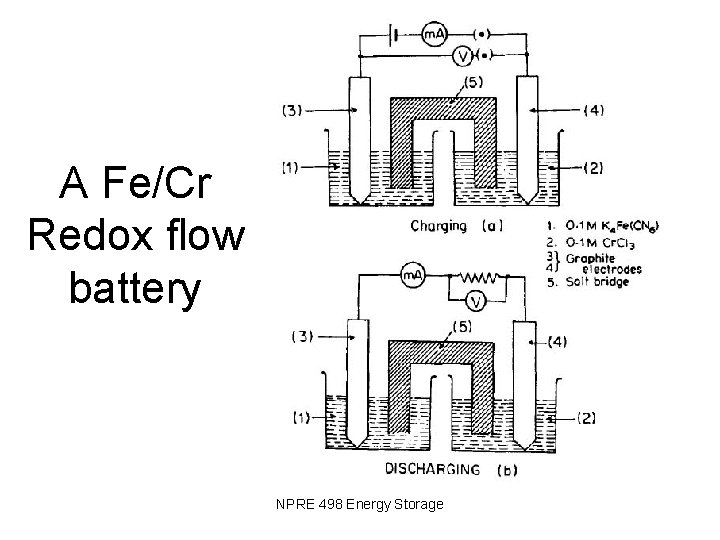
A Fe/Cr Redox flow battery NPRE 498 Energy Storage
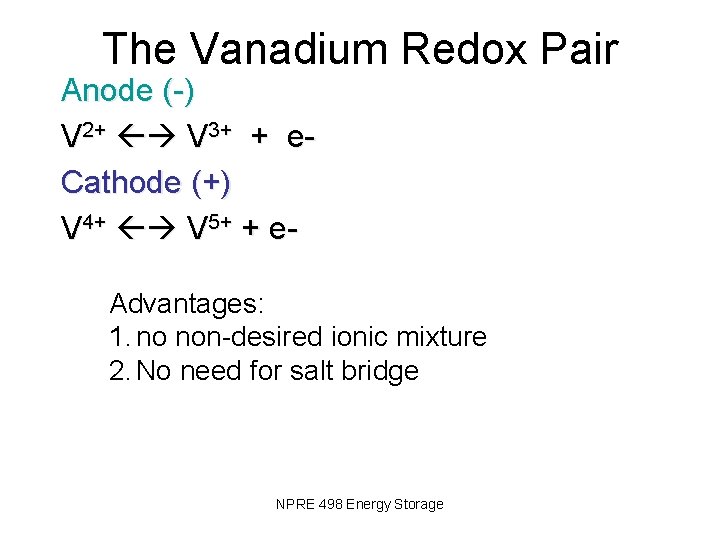
The Vanadium Redox Pair Anode (-) V 2+ V 3+ + e. Cathode (+) V 4+ V 5+ + e- Advantages: 1. no non-desired ionic mixture 2. No need for salt bridge NPRE 498 Energy Storage
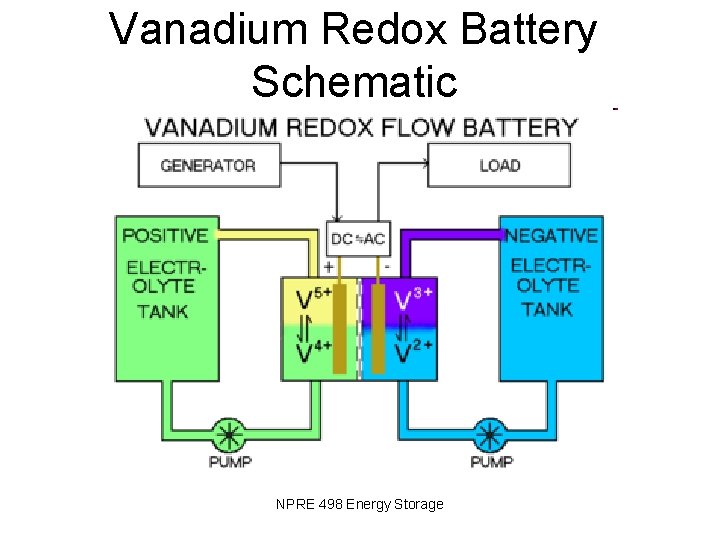
Vanadium Redox Battery Schematic NPRE 498 Energy Storage
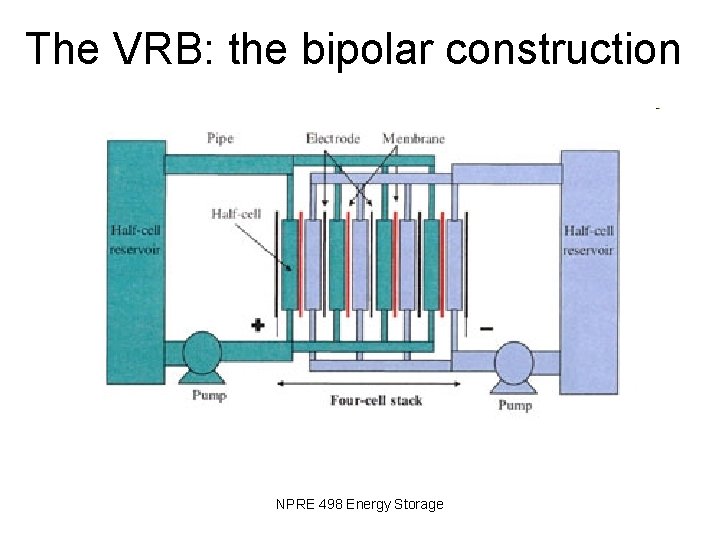
The VRB: the bipolar construction NPRE 498 Energy Storage

VRB: Real system NPRE 498 Energy Storage
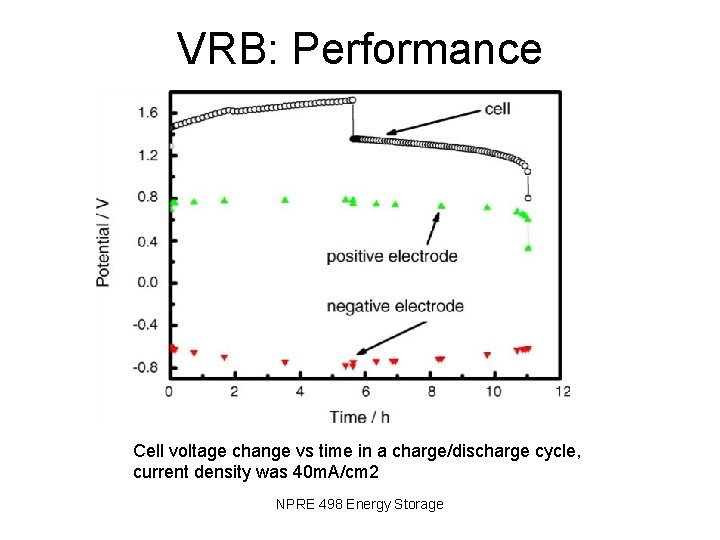
VRB: Performance Cell voltage change vs time in a charge/discharge cycle, current density was 40 m. A/cm 2 NPRE 498 Energy Storage
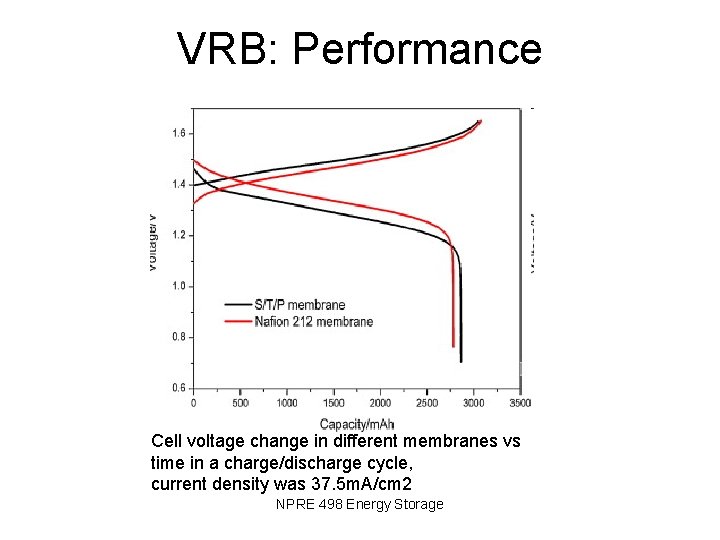
VRB: Performance Cell voltage change in different membranes vs time in a charge/discharge cycle, current density was 37. 5 m. A/cm 2 NPRE 498 Energy Storage
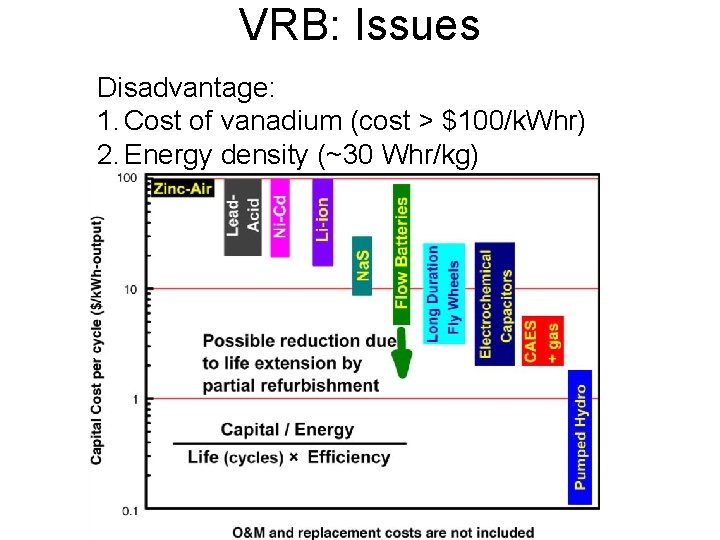
VRB: Issues Disadvantage: 1. Cost of vanadium (cost > $100/k. Whr) 2. Energy density (~30 Whr/kg) NPRE 498 Energy Storage
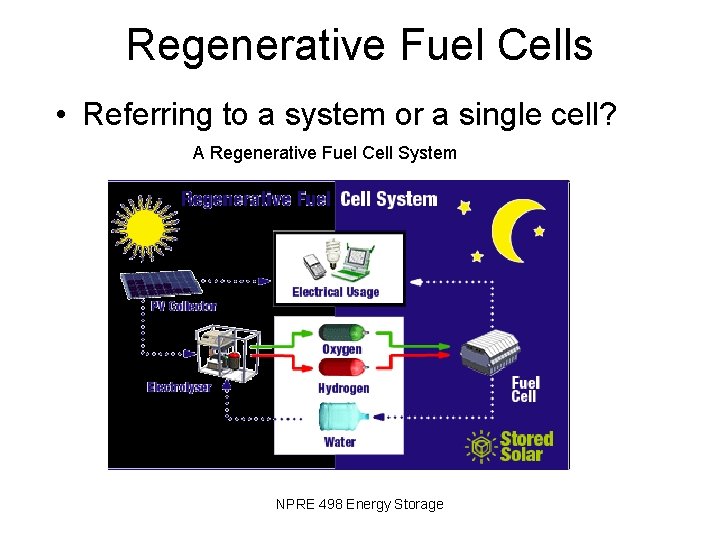
Regenerative Fuel Cells • Referring to a system or a single cell? A Regenerative Fuel Cell System NPRE 498 Energy Storage
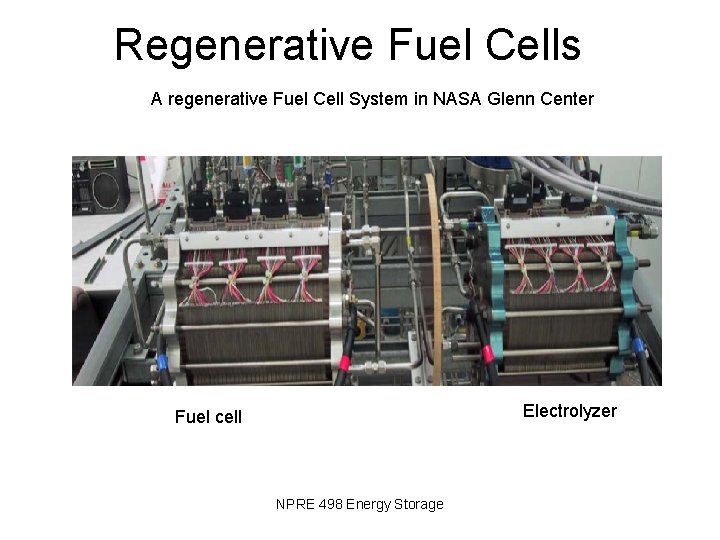
Regenerative Fuel Cells A regenerative Fuel Cell System in NASA Glenn Center Electrolyzer Fuel cell NPRE 498 Energy Storage
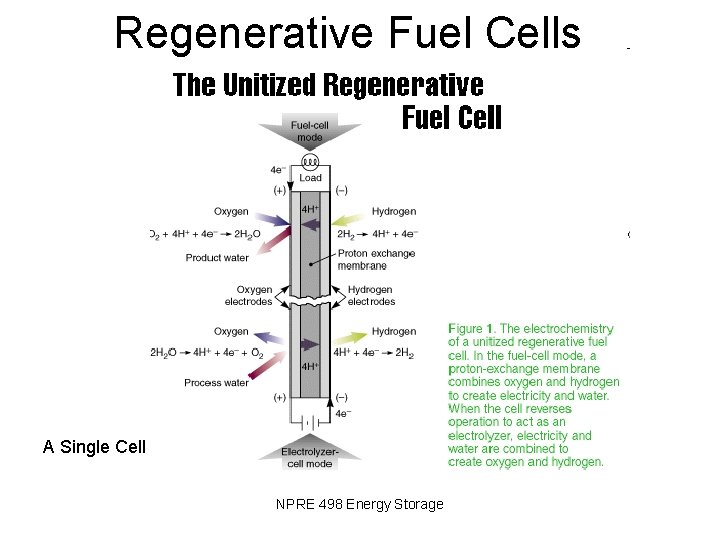
Regenerative Fuel Cells A Single Cell NPRE 498 Energy Storage
Regenerative Fuel Cells • But there is a big catch: • Hydrophobicity vs Hydrophilicity • Conflicting requirement in two modes for a gas phase product/reactant combination NPRE 498 Energy Storage

Regenerative Fuel Cells • • • All liquid RFC A bit like Redox flow battery Potentially higher energy density Kinetics is generally slower Example, Na. BH 4/H 2 O 2 NPRE 498 Energy Storage
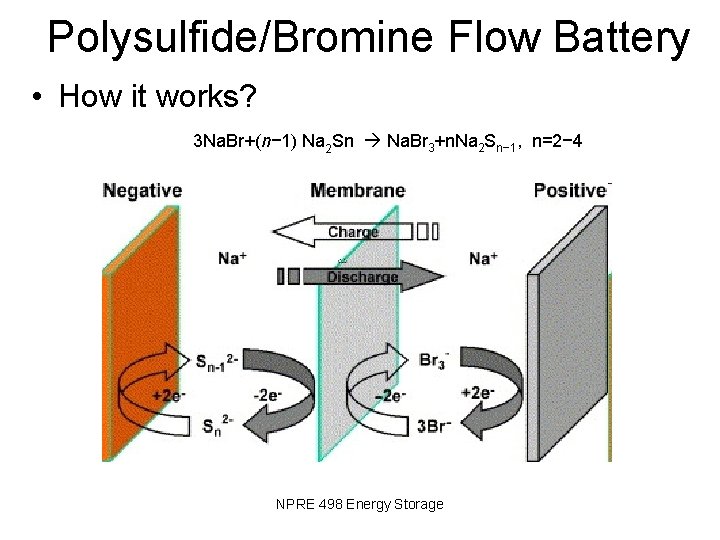
Polysulfide/Bromine Flow Battery • How it works? 3 Na. Br+(n− 1) Na 2 Sn Na. Br 3+n. Na 2 Sn− 1, n=2− 4 NPRE 498 Energy Storage
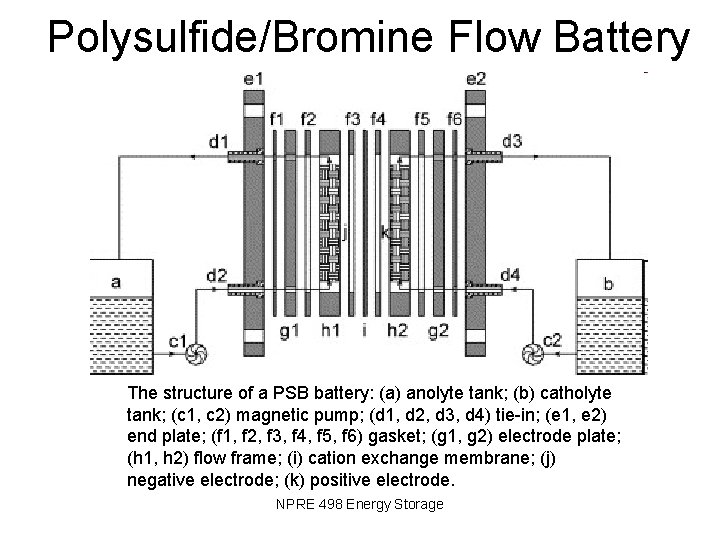
Polysulfide/Bromine Flow Battery The structure of a PSB battery: (a) anolyte tank; (b) catholyte tank; (c 1, c 2) magnetic pump; (d 1, d 2, d 3, d 4) tie-in; (e 1, e 2) end plate; (f 1, f 2, f 3, f 4, f 5, f 6) gasket; (g 1, g 2) electrode plate; (h 1, h 2) flow frame; (i) cation exchange membrane; (j) negative electrode; (k) positive electrode. NPRE 498 Energy Storage
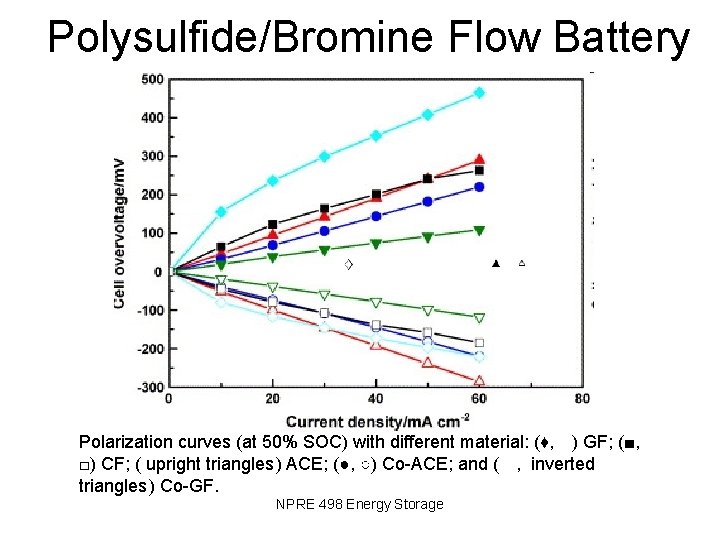
Polysulfide/Bromine Flow Battery Polarization curves (at 50% SOC) with different material: (♦, ) GF; (■, □) CF; ( upright triangles ) ACE; (●, ○) Co-ACE; and ( , inverted triangles ) Co-GF. NPRE 498 Energy Storage
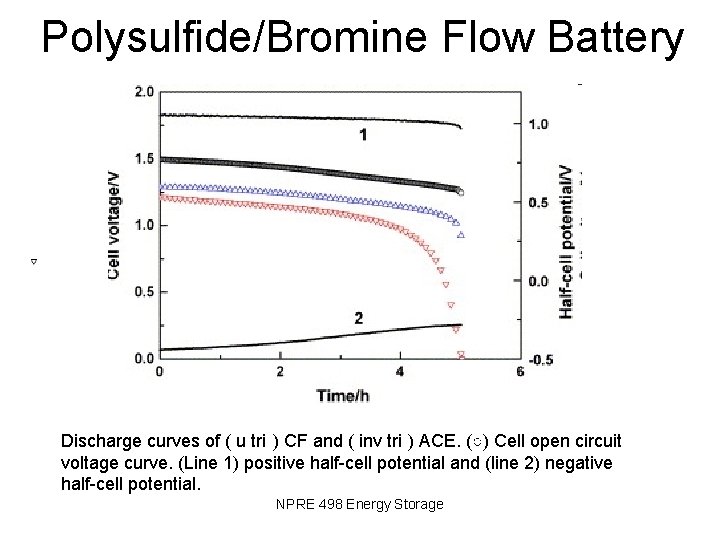
Polysulfide/Bromine Flow Battery Discharge curves of ( u tri ) CF and ( inv tri ) ACE. (○) Cell open circuit voltage curve. (Line 1) positive half-cell potential and (line 2) negative half-cell potential. NPRE 498 Energy Storage
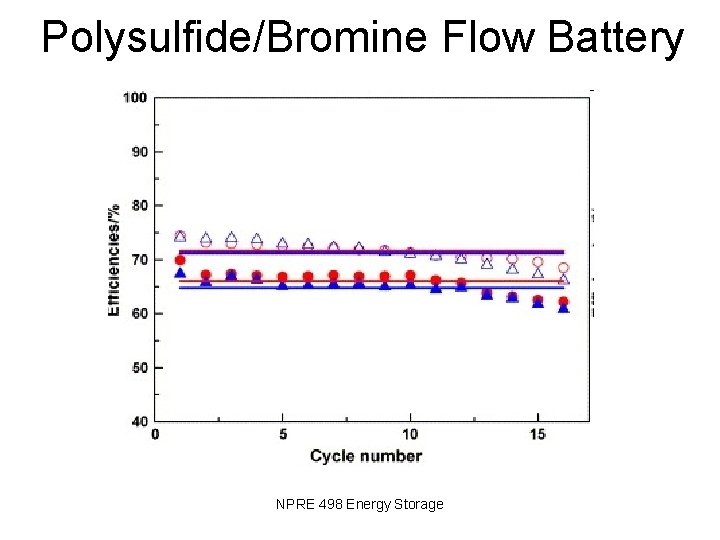
Polysulfide/Bromine Flow Battery NPRE 498 Energy Storage
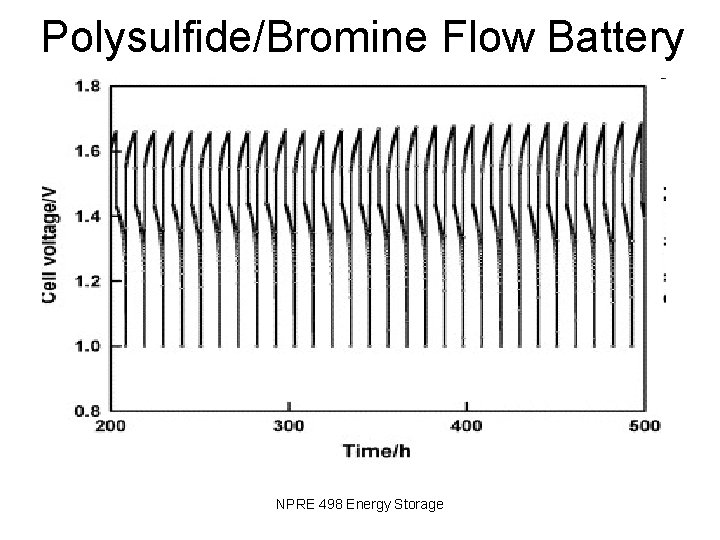
Polysulfide/Bromine Flow Battery NPRE 498 Energy Storage
Polysulfide/Bromine Flow Battery • Advantages – Low cost – Fast kinetics • Disadvantages – Cross-over – Poor stability NPRE 498 Energy Storage
- Slides: 22