Redox equations and disproportionation 01 January 2022 Redox





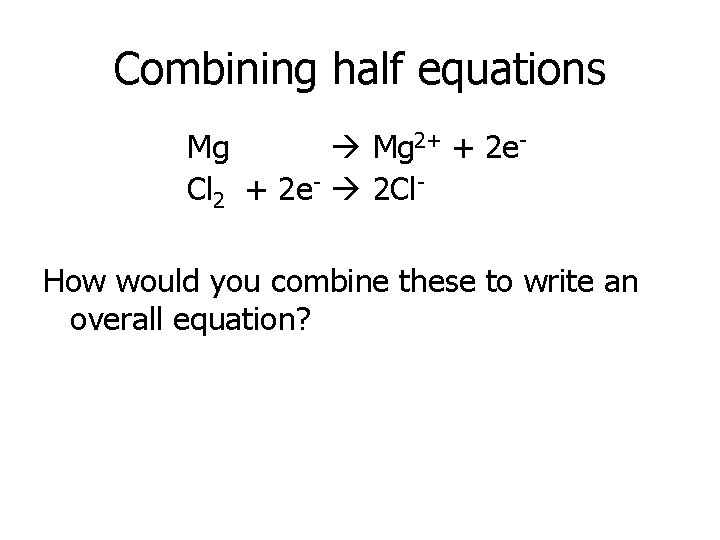



- Slides: 19

Redox equations and disproportionation 01 January 2022

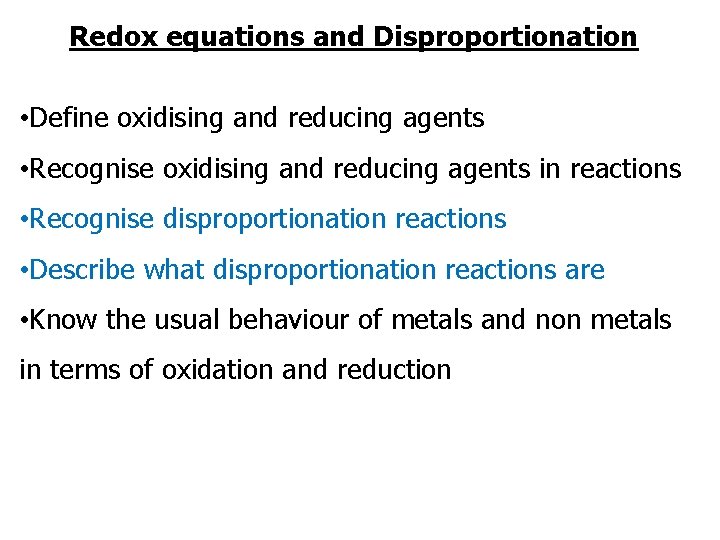
Redox equations and Disproportionation • Define oxidising and reducing agents • Recognise oxidising and reducing agents in reactions • Recognise disproportionation reactions • Describe what disproportionation reactions are • Know the usual behaviour of metals and non metals in terms of oxidation and reduction

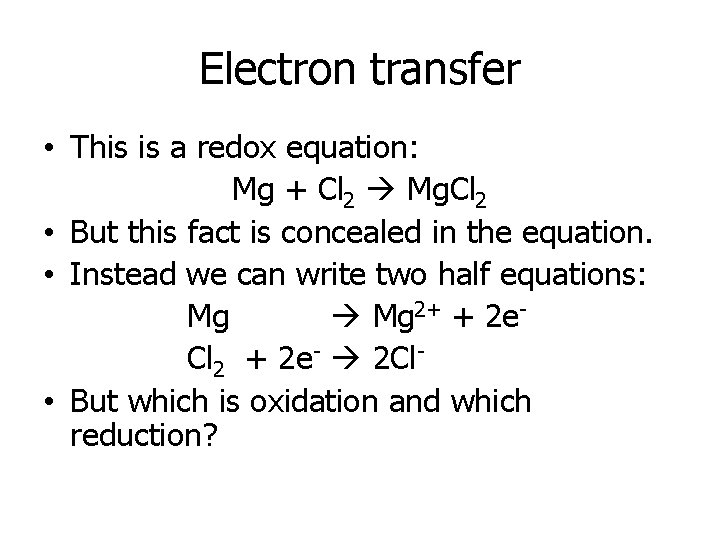
Electron transfer • This is a redox equation: Mg + Cl 2 Mg. Cl 2 • But this fact is concealed in the equation. • Instead we can write two half equations: Mg 2+ + 2 e. Cl 2 + 2 e- 2 Cl • But which is oxidation and which reduction?

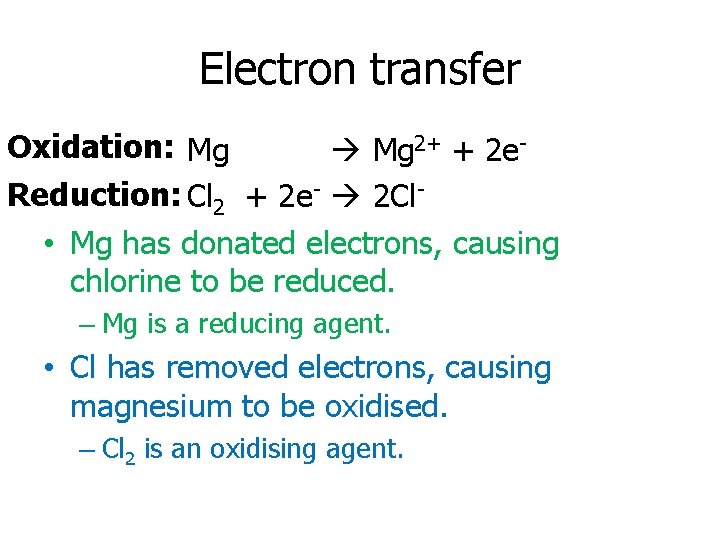
Electron transfer Oxidation: Mg 2+ + 2 e. Reduction: Cl 2 + 2 e- 2 Cl • Mg has donated electrons, causing chlorine to be reduced. – Mg is a reducing agent. • Cl has removed electrons, causing magnesium to be oxidised. – Cl 2 is an oxidising agent.

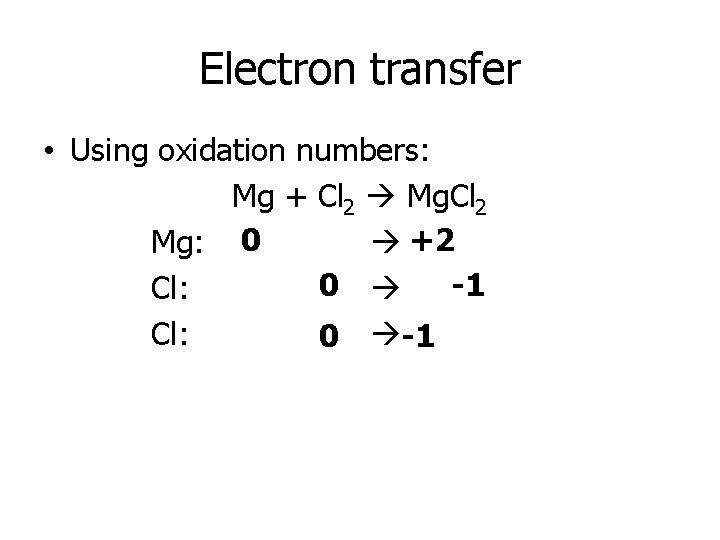
Electron transfer • Using oxidation numbers: Mg + Cl 2 Mg. Cl 2 Mg: 0 +2 0 -1 Cl: 0 -1

Metals with acid • The reactions of metals with acids are redox reactions. • The metal loses electrons to form a positive ion so it is _____ed. • The hydrogen in the acid (H+) is _____ed forming hydrogen gas.

• In general what charged ions do metals form? • Positive ions • How do they form these? • Losing electrons • Leading to an increase in oxidation numbers (becomes more positive)

• In general what do non metals do? • Form negative ions • How do they form these? • Gaining electrons • Leading to decrease in oxidation numbers (becomes more negative)
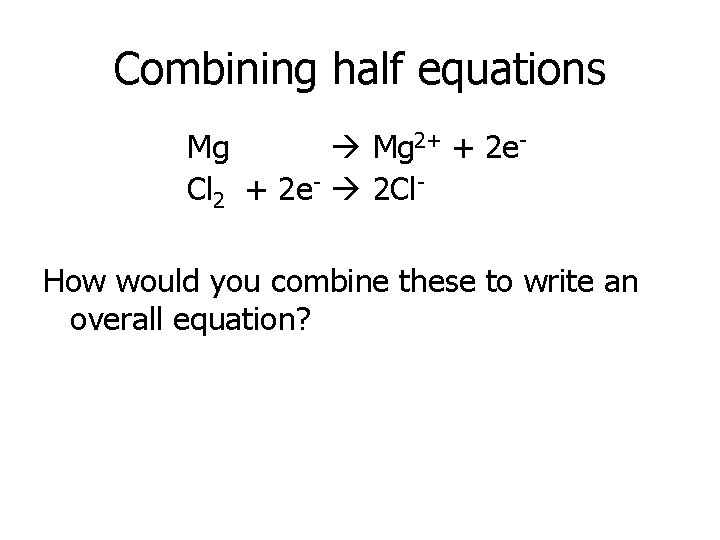
Combining half equations Mg 2+ + 2 e. Cl 2 + 2 e- 2 Cl. How would you combine these to write an overall equation?

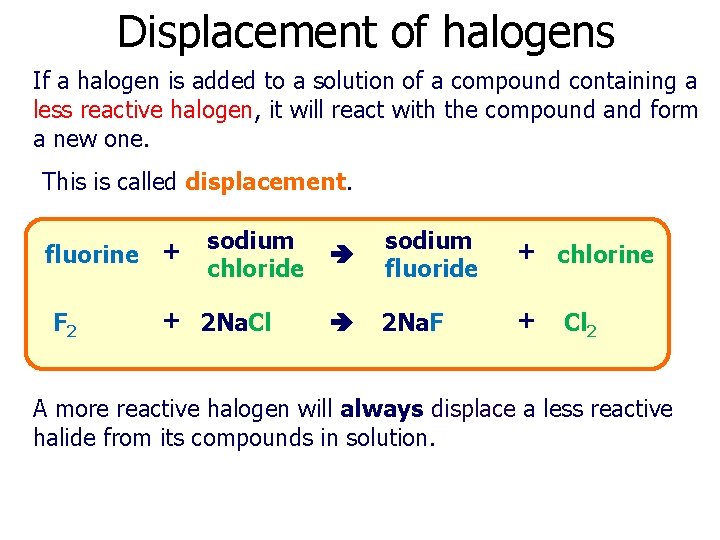
Displacement of halogens If a halogen is added to a solution of a compound containing a less reactive halogen, it will react with the compound and form a new one. This is called displacement. fluorine + F 2 sodium chloride + 2 Na. Cl sodium fluoride + chlorine 2 Na. F + Cl 2 A more reactive halogen will always displace a less reactive halide from its compounds in solution.

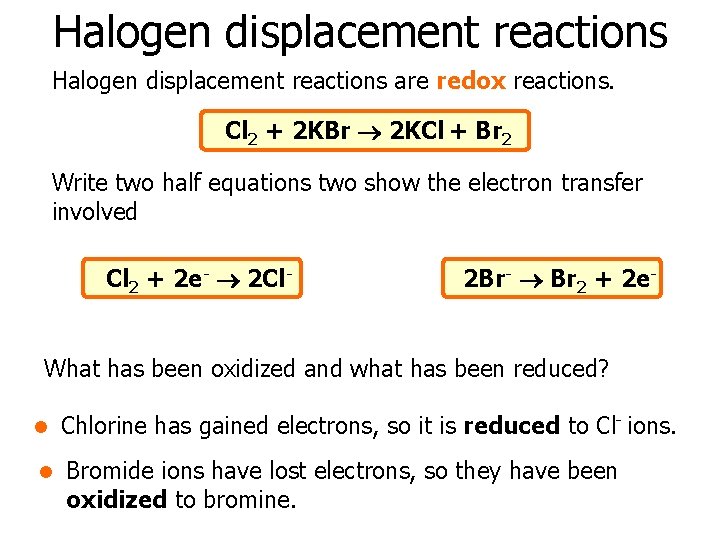
Halogen displacement reactions are redox reactions. Cl 2 + 2 KBr 2 KCl + Br 2 Write two half equations two show the electron transfer involved Cl 2 + 2 e- 2 Cl- 2 Br- Br 2 + 2 e- What has been oxidized and what has been reduced? l Chlorine has gained electrons, so it is reduced to Cl- ions. l Bromide ions have lost electrons, so they have been oxidized to bromine.

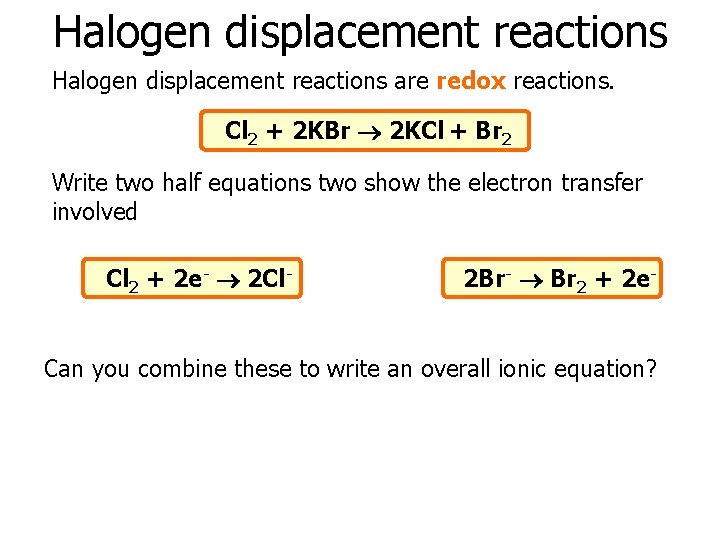
Halogen displacement reactions are redox reactions. Cl 2 + 2 KBr 2 KCl + Br 2 Write two half equations two show the electron transfer involved Cl 2 + 2 e- 2 Cl- 2 Br- Br 2 + 2 e- Can you combine these to write an overall ionic equation?

Challenge work Redox equations exam questions Complete worksheet Combining half equations to write full equations Page 5 starter for 10

Redox equations and Disproportionation • Define oxidising and reducing agents • Recognise oxidising and reducing agents in reactions • Recognise disproportionation reactions • Describe what disproportionation reactions are • Know the usual behaviour of metals and non metals in terms of oxidation and reduction

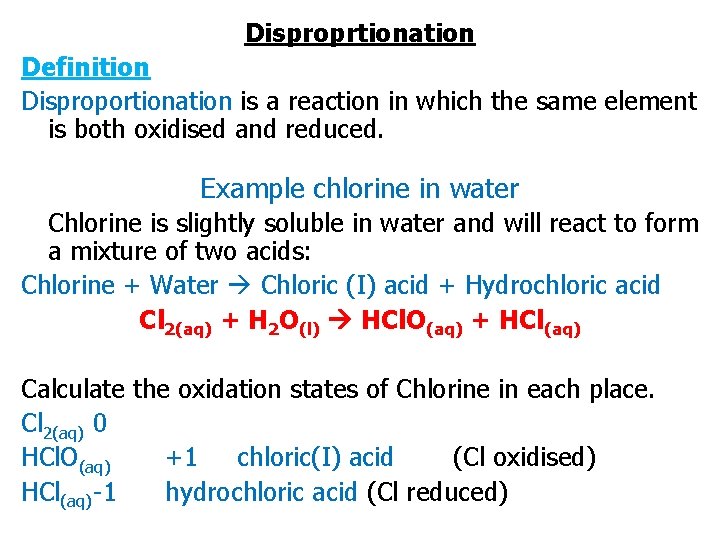
Disproprtionation Definition Disproportionation is a reaction in which the same element is both oxidised and reduced. Example chlorine in water Chlorine is slightly soluble in water and will react to form a mixture of two acids: Chlorine + Water Chloric (I) acid + Hydrochloric acid Cl 2(aq) + H 2 O(l) HCl. O(aq) + HCl(aq) Calculate the oxidation states of Chlorine in each place. Cl 2(aq) 0 HCl. O(aq) +1 chloric(I) acid (Cl oxidised) HCl(aq) -1 hydrochloric acid (Cl reduced)

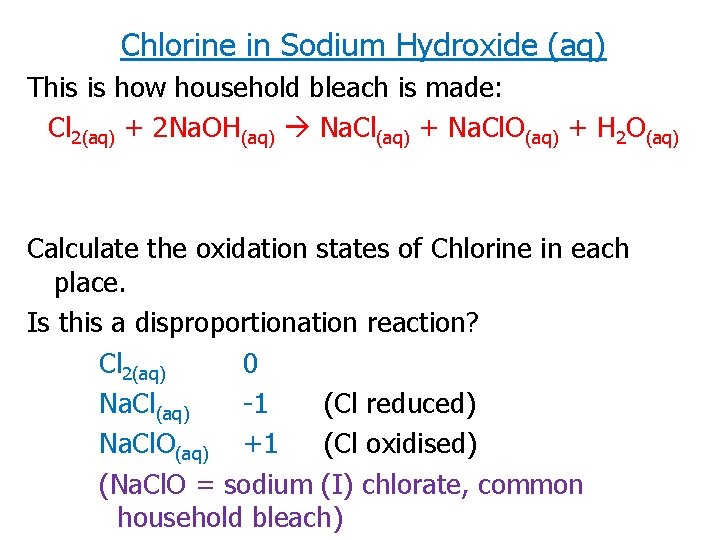
Chlorine in Sodium Hydroxide (aq) This is how household bleach is made: Cl 2(aq) + 2 Na. OH(aq) Na. Cl(aq) + Na. Cl. O(aq) + H 2 O(aq) Calculate the oxidation states of Chlorine in each place. Is this a disproportionation reaction? Cl 2(aq) 0 Na. Cl(aq) -1 (Cl reduced) Na. Cl. O(aq) +1 (Cl oxidised) (Na. Cl. O = sodium (I) chlorate, common household bleach)

Exam questions Q 3 redox eq Q 5 redox eq Q 7 redox eq

Redox equations and Disproportionation • Define oxidising and reducing agents • Recognise oxidising and reducing agents in reactions • Recognise disproportionation reactions • Describe what disproportionation reactions are • Know the usual behaviour of metals and non metals in terms of oxidation and reduction

Redox equations and Disproportionation • Define oxidising and reducing agents • Recognise oxidising and reducing agents in reactions • Recognise disproportionation reactions • Describe what disproportionation reactions are • Know the usual behaviour of metals and non metals in terms of oxidation and reduction