RED CELL STRUCTURE The RBC is 8 micrometer
RED CELL STRUCTURE • The RBC is 8 micrometer in diameter, with a flexible biconcave disc. • The red cell membrane comprises of a lipid bilayer, integral membrane proteins and a membrane skeleton. • Approximately 50% of the membrane is protein, 20% phospholipids, 20% cholesterol and 10% carbohydrate(cho).

• CHO occur only on the external surf while proteins are either peripheral or integral, penetrating the lipid bilayer. • Some proteins have been numbered according to their mobility on gel electrophoresis e. g Band 3, protein 4. 1, 4. 2 • The membrane skeleton is formed by structural proteins that include alpha and beta spectrin, ankyrin, protien 4. 1 and actin

• These protein form a horizontal lattice on the internal side of the red cell memb and are important in maintaining the biconcave shape. • Spectrin is the most abundant and consists of two chains alpha and beta, wound around each other to form heterodimers which then self associate head to form tetramers. • These tetramers are linked at the tail end to actin and are attached to protein band 4. 1.

• Beta spectrin chains attach to ankyrin which connect to band 3, the transmembrane protein that acts act as an anion channel, protein 4. 2 enhance this interaction. • Defects of the proteins may explain some of the abnormalities of the RBC memb (hereditary spherocytosis and hereditary elliptocytosis), while alterations in lipid composition may be associated with other membrane abnormalities

RED BLOOD CELL FUNCTION • With the help of Hb it carries oxygen from the lungs to the tissues and carbon dioxide from the tissues to the lungs. • It can generate energy as adenosine triphosphate(ATP) by the anaerobic glycolytic pathway - ATP provides energy for maintenance of red cell volume, shape and flexibility

• It can generate nicotinamide adenine dinucleotide(NADH), a reducing agent by the anaerobic glycolytic pathway. • NADH is needed by the enzyme methaemaglobin reductase to reduce oxidized Hb containing ferric Fe to functionally active redced haemoglobin.
• Reduced NADPH produced by hexose monophosphate shunt is linked with glutathione which maintain sulphydril group intact in the cell membrane • 2, 3 -DPG is important in the regulation of haemoglobin oxygen affinity.

HAEMOGLOBIN SYNTHESIS
• Haem synthesis occur mainly in the mitochondria by a series of biochemical reactions commencing with the condensation of glycine and succinyl coenzyme A which under the action of the key rate limiting enzyme ALA synthase. • Pyridoxal phosphate is a coenzyme for this reaction. • Ultimately , protoporphyrin combines with Fe in the ferrous state to form haem, each molecule of which combine with a globin chain made on the polyribosomes.
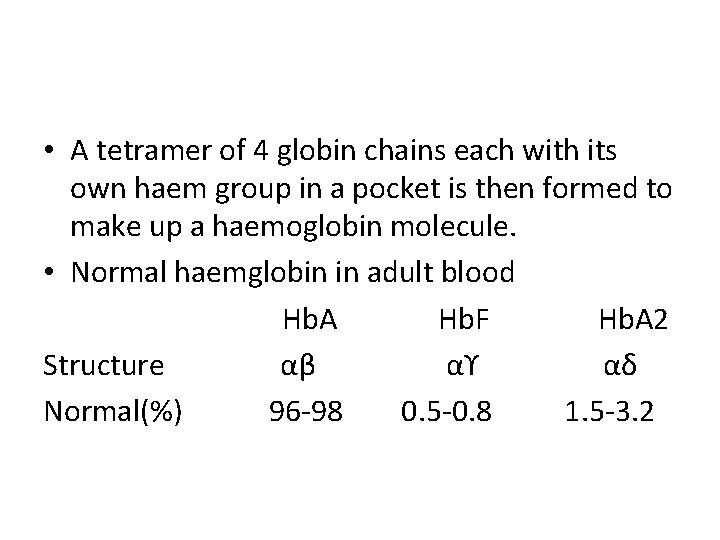
• A tetramer of 4 globin chains each with its own haem group in a pocket is then formed to make up a haemoglobin molecule. • Normal haemglobin in adult blood Hb. A Hb. F Hb. A 2 Structure αβ αϒ αδ Normal(%) 96 -98 0. 5 -0. 8 1. 5 -3. 2

FUNCTION OF HAEMOGLOBIN • The RBC carries oxygen from the lungs to the tissues and return carbon dioxide to the lungs, this function is carried out with the help of haemoglobin. • In Hb-oxygen dissociation curve, the partial pressure of oxygen at which Hb is half saturated with oxygen is 26. 6 mm. Hg. • With increased affinity of oxygen, the curve shifts to the left, while with decreased affinity for oxygen , the curve shifts to the right.

• High concentration of 2, 3 -DPG, Hydrogen ion or CO 2 and Hb S shifts the curve to the right(oxygen is given up easily), whereas Hb. F, decreased hydrogen ion or CO 2 and decreased 2, 3 -DPG shift the curve to the left(they give up oxygen less readily than normal).
- Slides: 12