Recycle Bypass and Purge Streams Definition of each
Recycle, Bypass and Purge Streams Definition of each term Why do we use recycle streams ? Examples: recycled cans, newspapers, exit catalyst, and the reflux stream in a distillation column Systems are operating in the steady state The values of the flows are constant The steps in the analysis and solution are exactly the same as before. Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004
For reaction systems: -We use either atomic balances or the stoichiometric equations. -We carry component balances for components not involved in the reaction -For component balances around the reactor, we use the steady state general MB equation : Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004
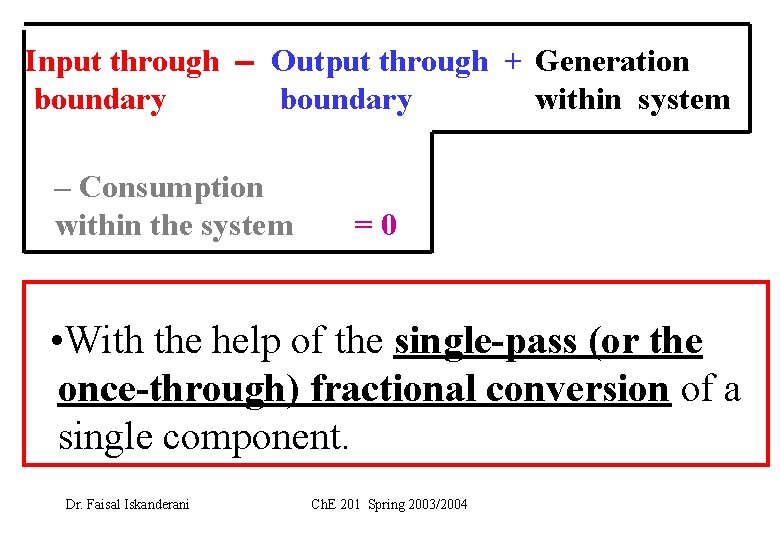
Input through Output through + Generation boundary within system – Consumption within the system =0 • With the help of the single-pass (or the once-through) fractional conversion of a single component. Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004
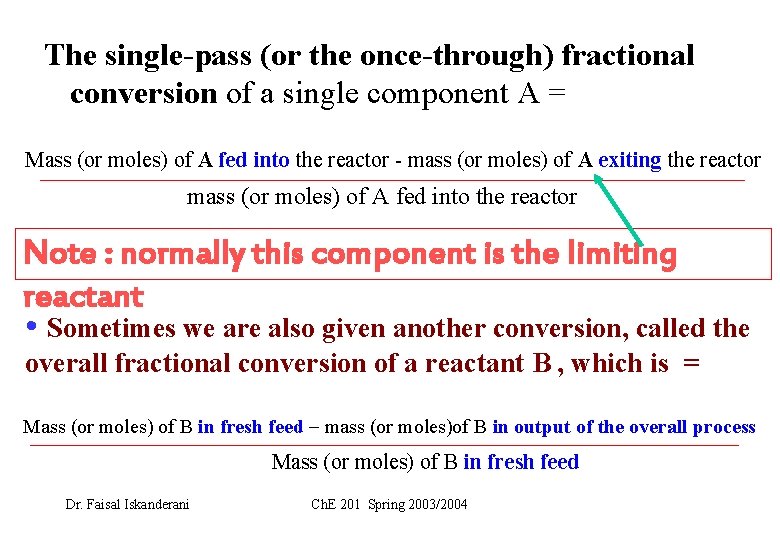
The single-pass (or the once-through) fractional conversion of a single component A = Mass (or moles) of A fed into the reactor - mass (or moles) of A exiting the reactor mass (or moles) of A fed into the reactor Note : normally this component is the limiting reactant • Sometimes we are also given another conversion, called the overall fractional conversion of a reactant B , which is = Mass (or moles) of B in fresh feed mass (or moles)of B in output of the overall process Mass (or moles) of B in fresh feed Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004
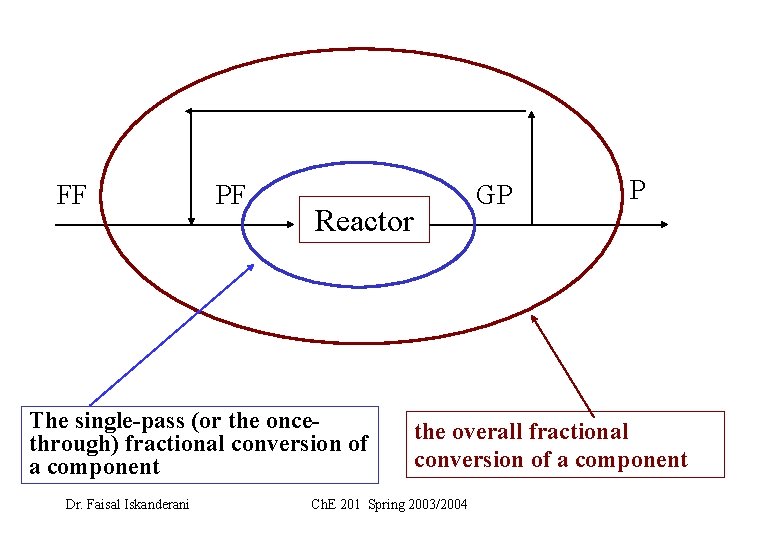
FF PF Reactor The single-pass (or the oncethrough) fractional conversion of a component Dr. Faisal Iskanderani GP P the overall fractional conversion of a component Ch. E 201 Spring 2003/2004
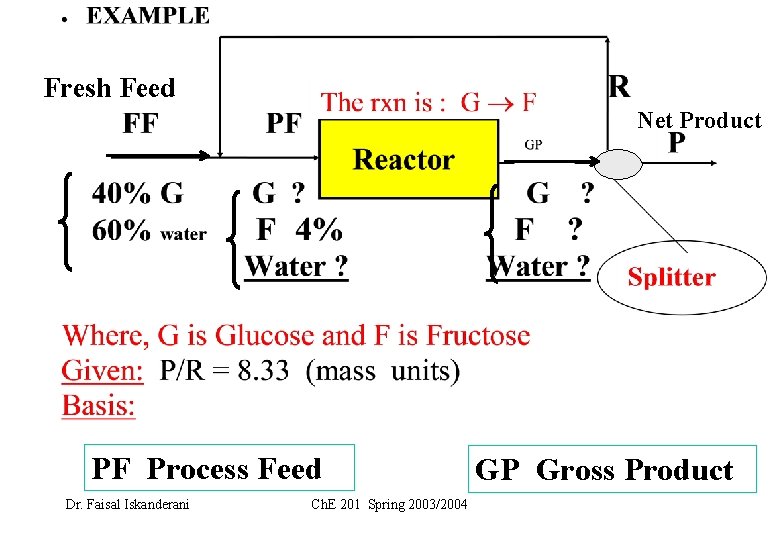
Fresh Feed Net Product PF Process Feed Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004 GP Gross Product

• We have three types of junction points: 1 - A Splitter where the stream leaving the reactor splits into 2 or more streams. The composition in all these streams is same. 2 - A Separator where the stream leaving the reactor enters into a separation process (such as distillation, extraction, etc. . ). The 2 or more streams leaving the separator have different compositions 3 - A mixing point where 2 streams or more join together. Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004
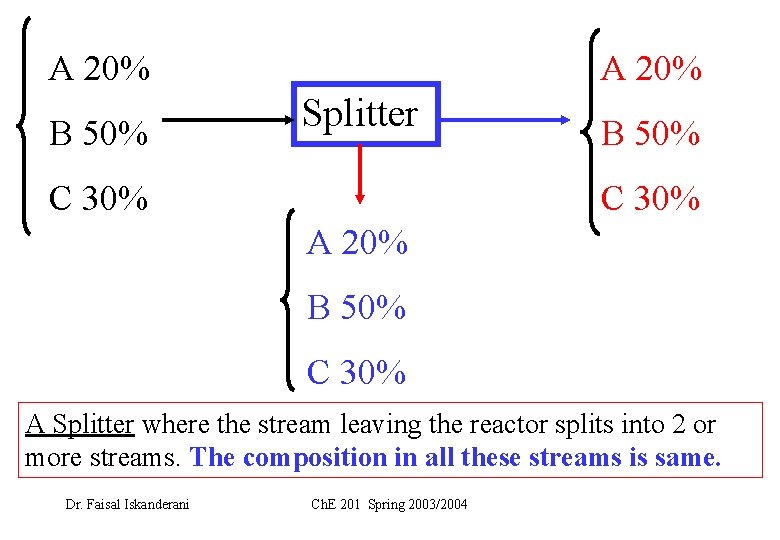
A 20% B 50% A 20% Splitter C 30% B 50% C 30% A 20% B 50% C 30% A Splitter where the stream leaving the reactor splits into 2 or more streams. The composition in all these streams is same. Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004

A 20% B 50% A 10% Separator C 30% B 75% C 15% A 70% B 28% C 2% A Separator where the stream leaving the reactor enters into a separation process (such as distillation, extraction, etc. . ). The 2 or more streams leaving the separator have different compositions Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004
Mixing pt Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004
Example Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004
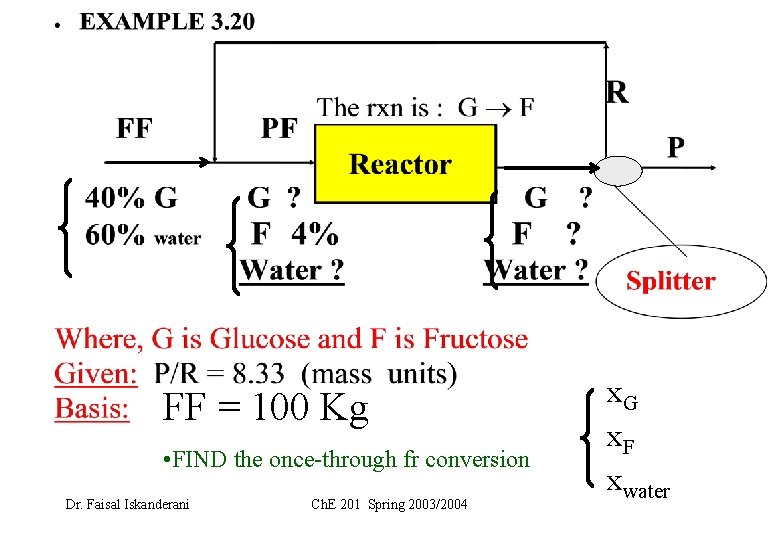
FF = 100 Kg • FIND the once-through fr conversion Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004 x. G x. F xwater

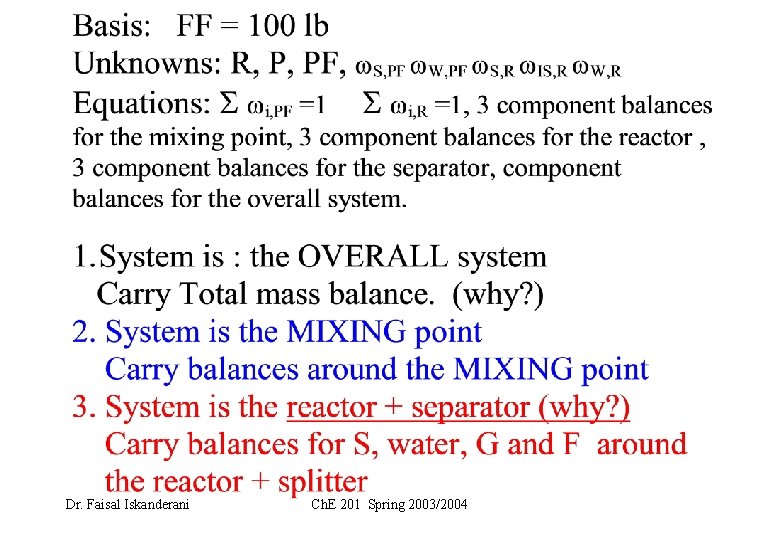
Basis: FF = 100 kg Let the once-through fractional conversion of G = f 1. System is : the OVERALL system Carry Total mass balance: Carry water component balance: (why? ) 2. System is the MIXING point Carry balances around the MIXING point 3. System is the reactor + splitter (why? ) Carry balances around the reactor + splitter Solve for the unknowns Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004
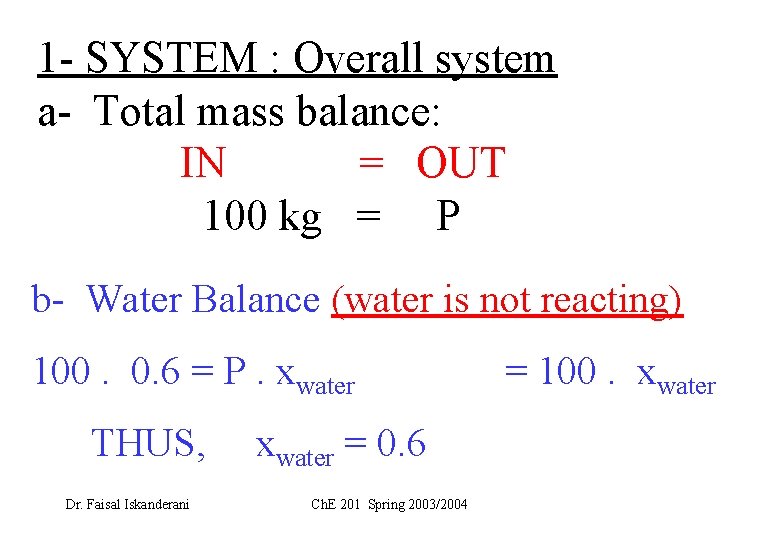
1 - SYSTEM : Overall system a- Total mass balance: IN = OUT 100 kg = P b- Water Balance (water is not reacting) 100. 0. 6 = P. xwater THUS, Dr. Faisal Iskanderani xwater = 0. 6 Ch. E 201 Spring 2003/2004 = 100. xwater
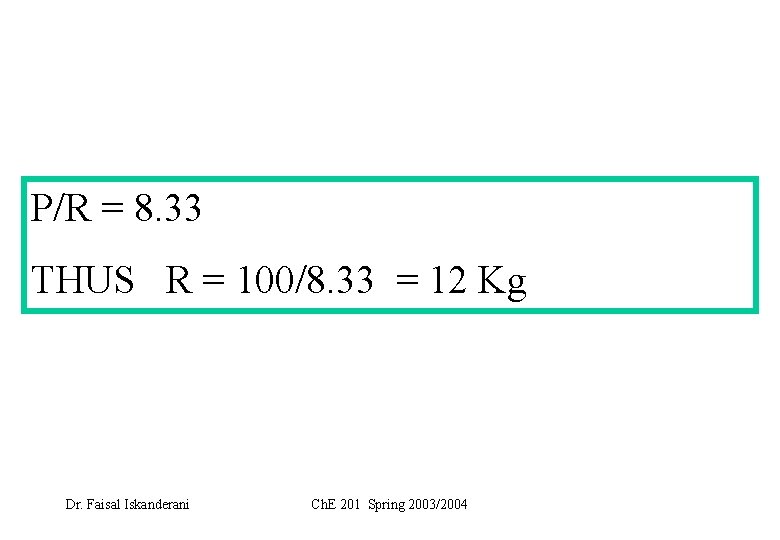
P/R = 8. 33 THUS R = 100/8. 33 = 12 Kg Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004
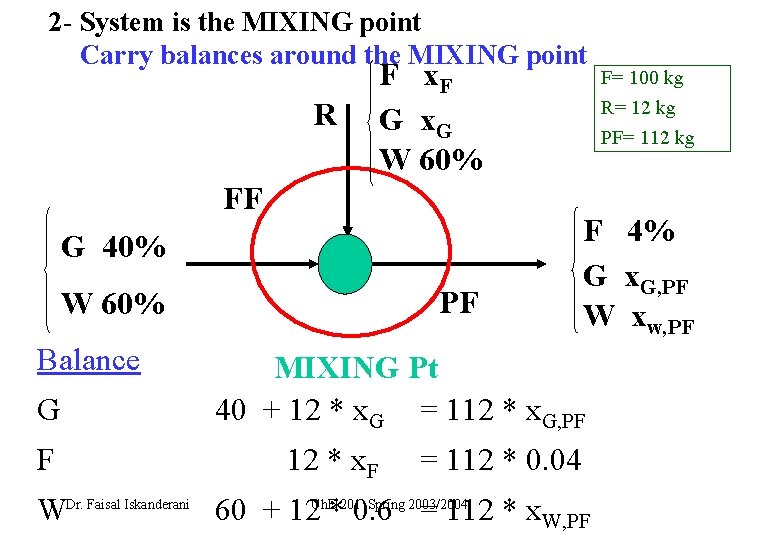
2 - System is the MIXING point Carry balances around the MIXING point R F x. F G x. G W 60% FF G 40% PF W 60% Balance G F R= 12 kg PF= 112 kg F 4% G x. G, PF W xw, PF MIXINGPt Pt MIXING 40 + 12 * x. G = 112 * x. G, PF 12 * x. F F= 100 kg = 112 * 0. 04 Spring 2003/2004 WDr. Faisal Iskanderani 60 + 12 Ch. E*2010. 6 = 112 * x. W, PF
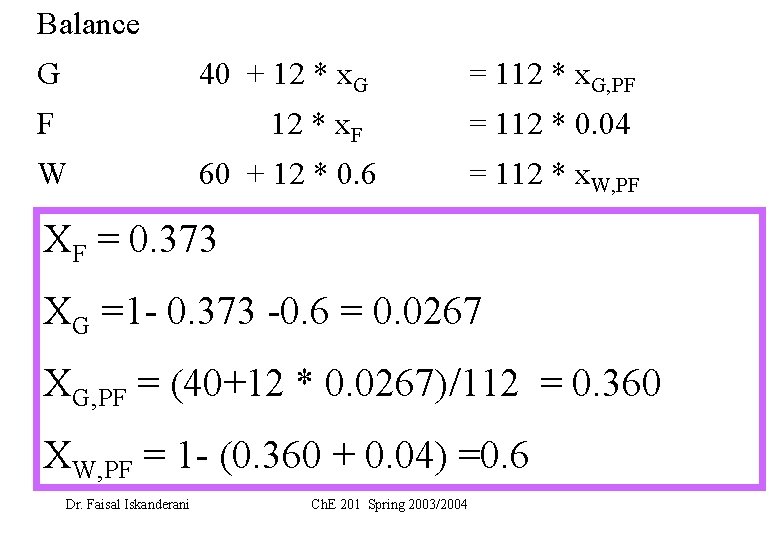
Balance G 40 + 12 * x. G = 112 * x. G, PF F 12 * x. F = 112 * 0. 04 W 60 + 12 * 0. 6 = 112 * x. W, PF XF = 0. 373 XG =1 - 0. 373 -0. 6 = 0. 0267 XG, PF = (40+12 * 0. 0267)/112 = 0. 360 XW, PF = 1 - (0. 360 + 0. 04) =0. 6 Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004
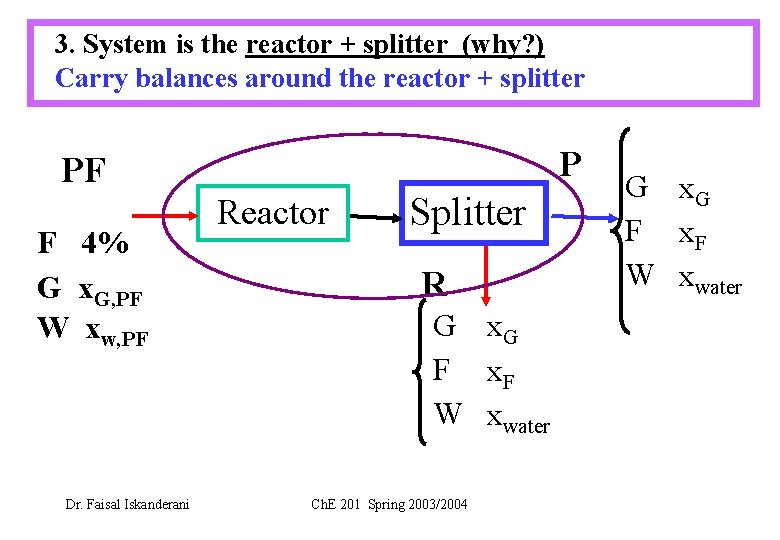
3. System is the reactor + splitter (why? ) Carry balances around the reactor + splitter P PF F 4% G x. G, PF W xw, PF Dr. Faisal Iskanderani Reactor Splitter R G x. G F x. F W xwater Ch. E 201 Spring 2003/2004 G x. G F x. F W xwater
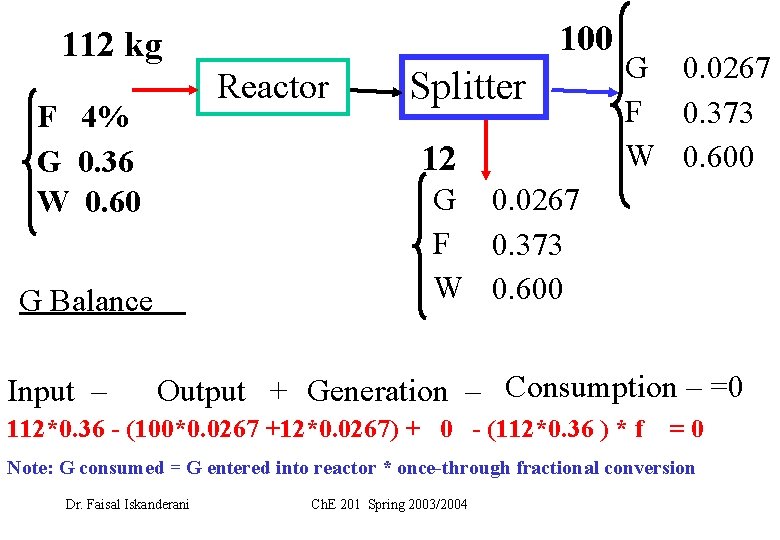
100 112 kg Reactor F 4% G 0. 36 W 0. 60 12 G 0. 0267 F 0. 373 W 0. 600 G Balance Input – Splitter G 0. 0267 F 0. 373 W 0. 600 Output + Generation – Consumption – =0 112*0. 36 - (100*0. 0267 +12*0. 0267) + 0 - (112*0. 36 ) * f =0 Note: G consumed = G entered into reactor * once-through fractional conversion Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004

112*0. 36 (100*0. 0267 +12*0. 0267) f= (112*0. 36 ) = 0. 93 Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004

New Example (take home) Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004

Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004
Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004
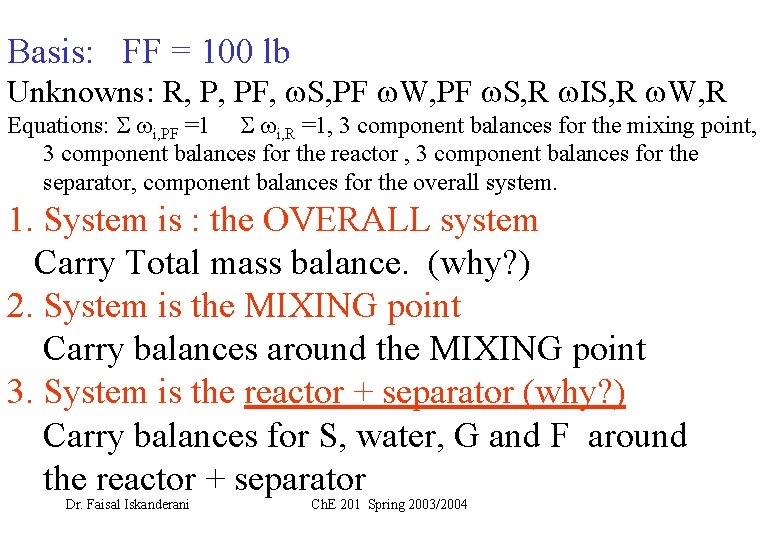
Basis: FF = 100 lb Unknowns: R, P, PF, S, PF W, PF S, R IS, R W, R Equations: i, PF =1 i, R =1, 3 component balances for the mixing point, 3 component balances for the reactor , 3 component balances for the separator, component balances for the overall system. 1. System is : the OVERALL system Carry Total mass balance. (why? ) 2. System is the MIXING point Carry balances around the MIXING point 3. System is the reactor + separator (why? ) Carry balances for S, water, G and F around the reactor + separator Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004

Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004

New Example Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004
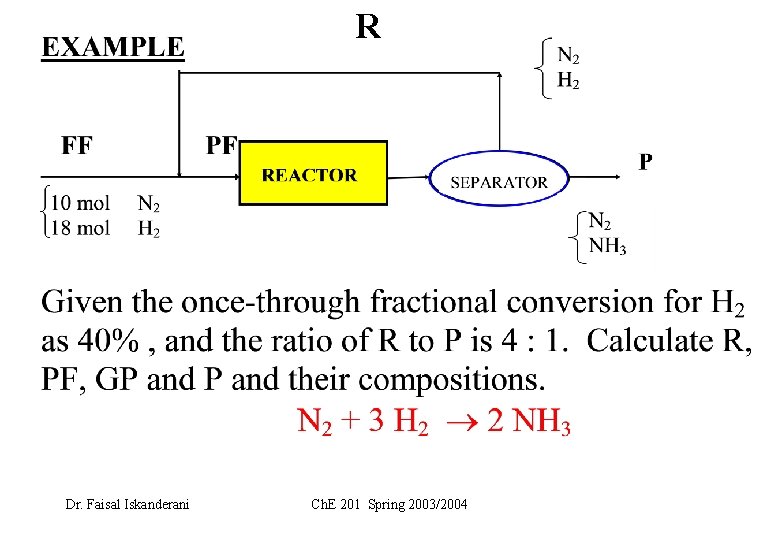
R Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004
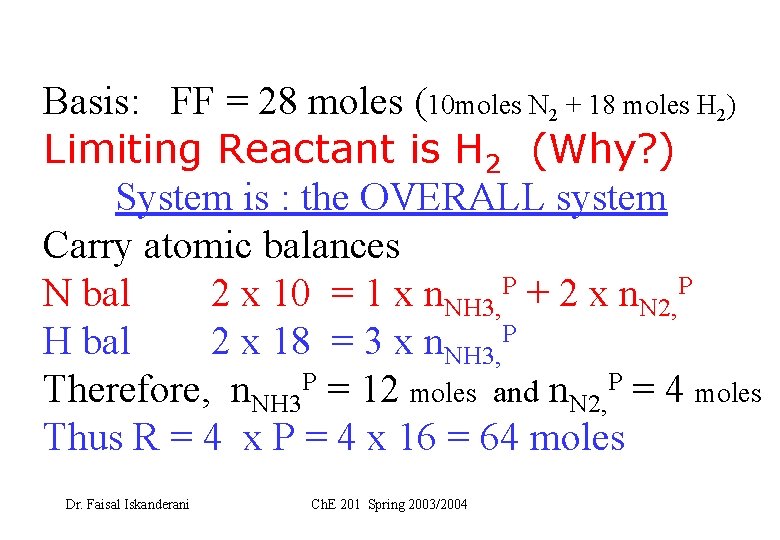
Basis: FF = 28 moles (10 moles N 2 + 18 moles H 2) Limiting Reactant is H 2 (Why? ) System is : the OVERALL system Carry atomic balances N bal 2 x 10 = 1 x n. NH 3, P + 2 x n. N 2, P H bal 2 x 18 = 3 x n. NH 3, P Therefore, n. NH 3 P = 12 moles and n. N 2, P = 4 moles Thus R = 4 x P = 4 x 16 = 64 moles Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004
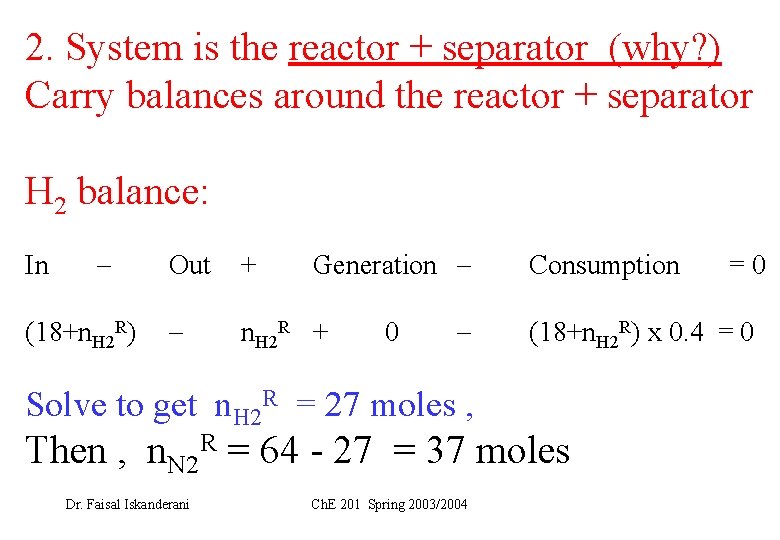
2. System is the reactor + separator (why? ) Carry balances around the reactor + separator H 2 balance: In (18+n. H 2 R) Generation Out + n. H 2 R + 0 Solve to get n. H 2 R = 27 moles , Consumption (18+n. H 2 R) x 0. 4 = 0 Then , n. N 2 R = 64 - 27 = 37 moles Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004 =0
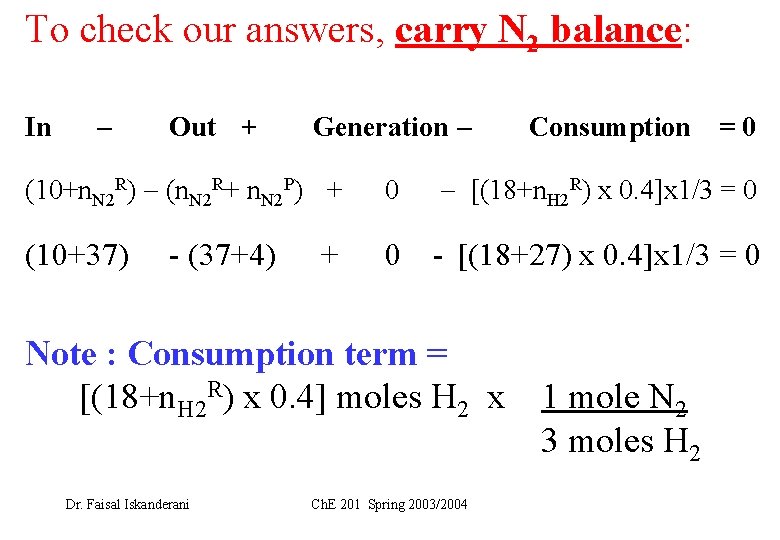
To check our answers, carry N 2 balance: In Out + Generation Consumption =0 (10+n. N 2 R) (n. N 2 R+ n. N 2 P) + 0 [(18+n. H 2 R) x 0. 4]x 1/3 = 0 (10+37) 0 - [(18+27) x 0. 4]x 1/3 = 0 - (37+4) + Note : Consumption term = [(18+n. H 2 R) x 0. 4] moles H 2 x Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004 1 mole N 2 3 moles H 2
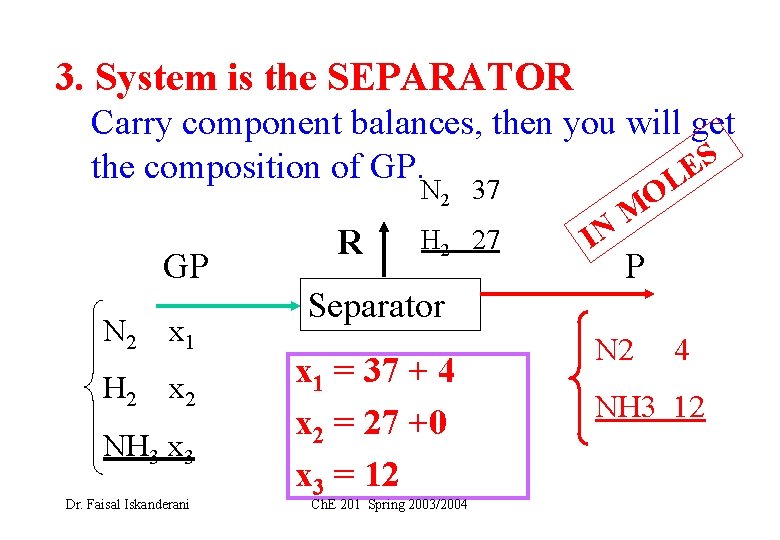
3. System is the SEPARATOR Carry component balances, then you will get S the composition of GP. LE N 2 37 GP N 2 x 1 H 2 x 2 NH 3 x 3 Dr. Faisal Iskanderani R H 2 27 Separator x 1 = 37 + 4 x 2 = 27 +0 x 3 = 12 Ch. E 201 Spring 2003/2004 IN O M P N 2 4 NH 3 12
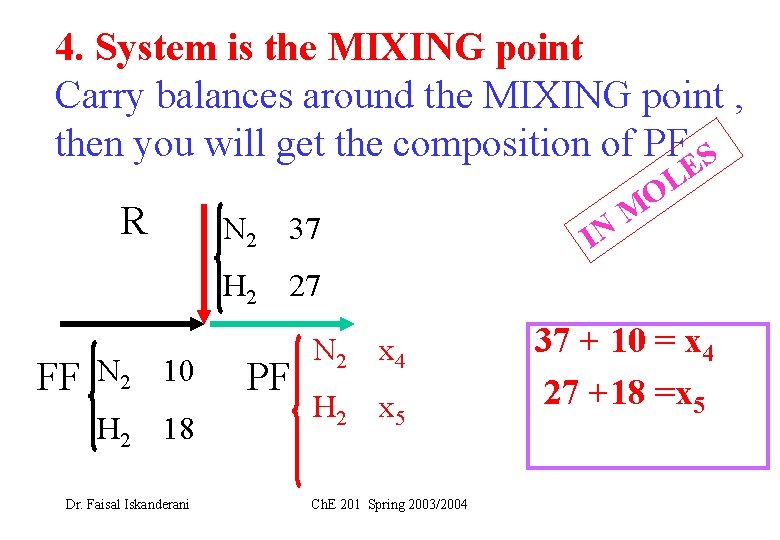
4. System is the MIXING point Carry balances around the MIXING point , then you will get the composition of PF S R N 2 37 IN E L O M H 2 27 FF N 2 10 H 2 18 Dr. Faisal Iskanderani PF N 2 x 4 H 2 x 5 Ch. E 201 Spring 2003/2004 37 + 10 = x 4 27 +18 =x 5

New Example Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004

A Purge : a stream bled off to remove an accumulation of inerts or unwanted material that might otherwise build up in the recycle stream R CO + 2 H 2 CH 3 OH Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004
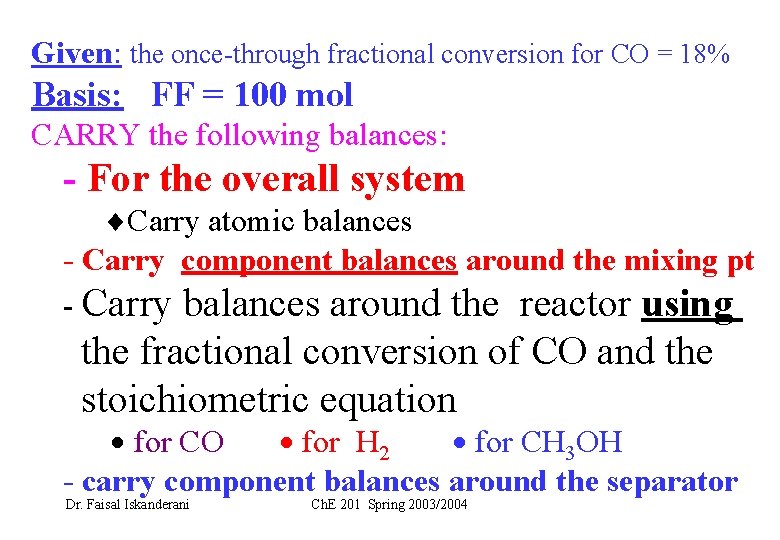
Given: the once-through fractional conversion for CO = 18% Basis: FF = 100 mol CARRY the following balances: - For the overall system Carry atomic balances - Carry component balances around the mixing pt - Carry balances around the reactor using the fractional conversion of CO and the stoichiometric equation for CO for H 2 for CH 3 OH - carry component balances around the separator Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004

1 - For the overall system Carry atomic balances Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004
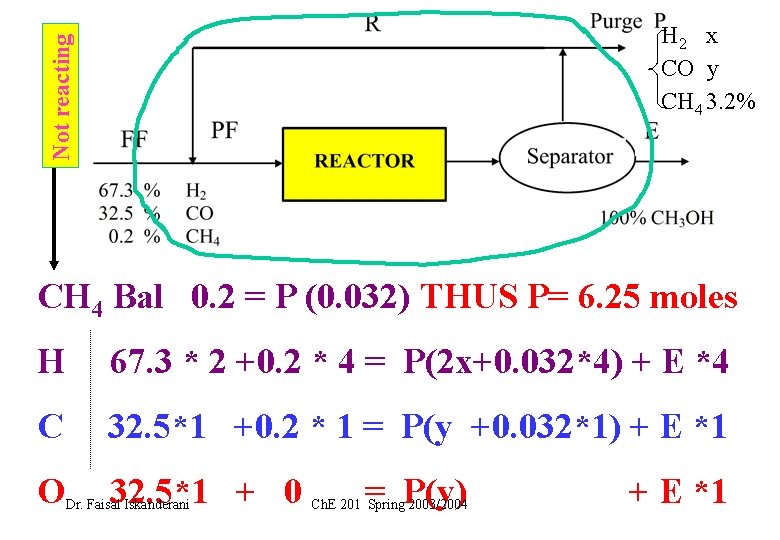
Not reacting H 2 x CO y CH 4 3. 2% CH 4 Bal 0. 2 = P (0. 032) THUS P= 6. 25 moles H 67. 3 * 2 +0. 2 * 4 = P(2 x+0. 032*4) + E *4 C 32. 5*1 +0. 2 * 1 = P(y +0. 032*1) + E *1 O 32. 5*1 + 0 Dr. Faisal Iskanderani = P(y) Ch. E 201 Spring 2003/2004 + E *1
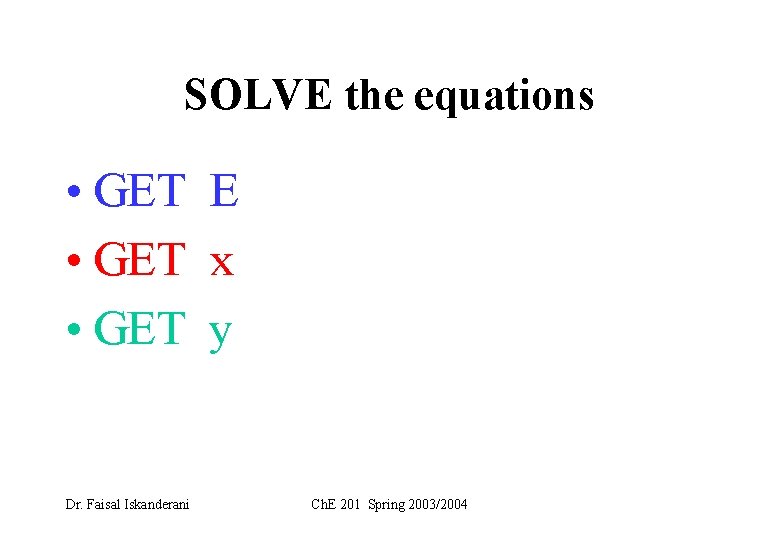
SOLVE the equations • GET E • GET x • GET y Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004
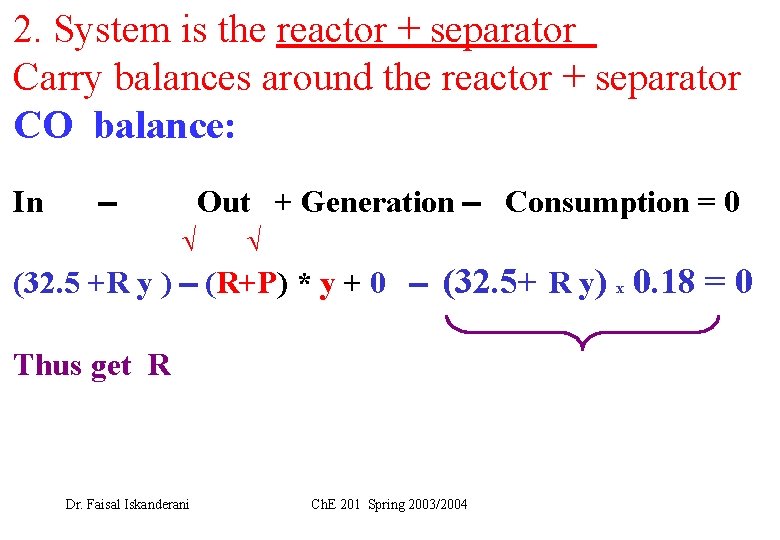
2. System is the reactor + separator Carry balances around the reactor + separator CO balance: In Out + Generation Consumption = 0 (32. 5 +R y ) (R+P) * y + 0 (32. 5+ R y) x 0. 18 = 0 Thus get R Dr. Faisal Iskanderani Ch. E 201 Spring 2003/2004
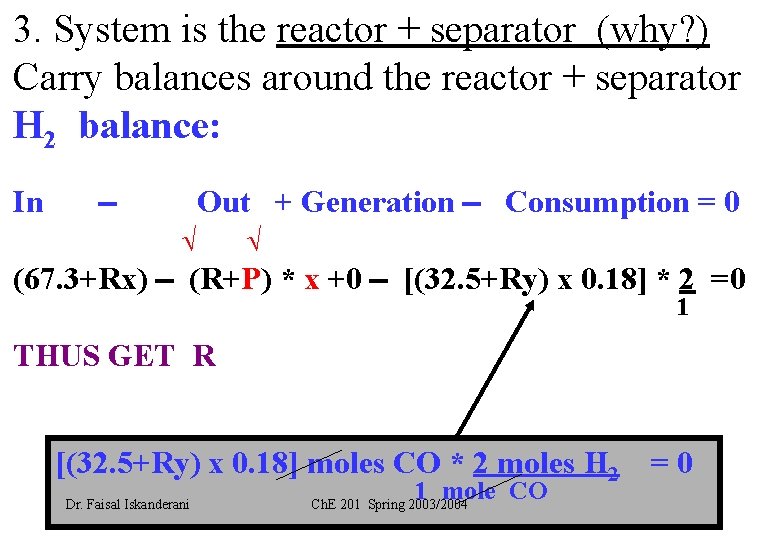
3. System is the reactor + separator (why? ) Carry balances around the reactor + separator H 2 balance: In Out + Generation Consumption = 0 (67. 3+Rx) (R+P) * x +0 [(32. 5+Ry) x 0. 18] * 2 =0 1 THUS GET R [(32. 5+Ry) x 0. 18] moles CO * 2 moles H 2 = 0 Dr. Faisal Iskanderani 1 mole CO Ch. E 201 Spring 2003/2004
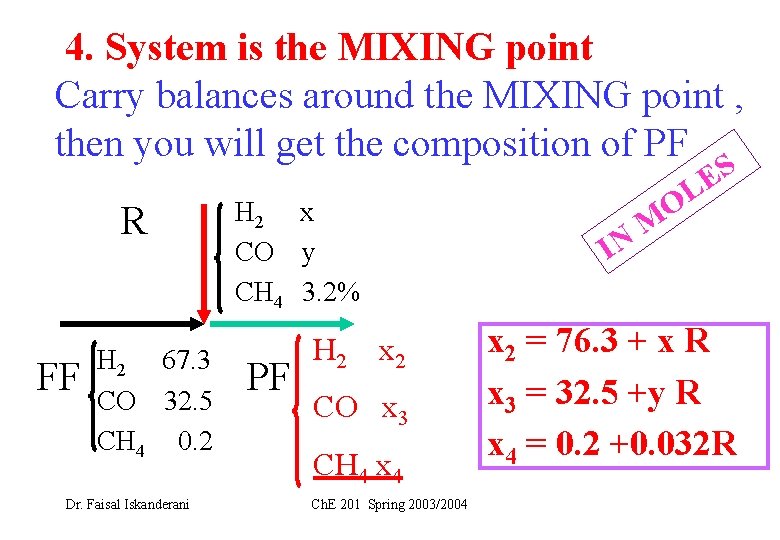
4. System is the MIXING point Carry balances around the MIXING point , then you will get the composition of PF R FF H 2 67. 3 CO 32. 5 CH 4 0. 2 Dr. Faisal Iskanderani H 2 x CO y CH 4 3. 2% PF H 2 x 2 CO x 3 CH 4 x 4 Ch. E 201 Spring 2003/2004 IN S E L M O x 2 = 76. 3 + x R x 3 = 32. 5 +y R x 4 = 0. 2 +0. 032 R
- Slides: 41