Reclassification of Medicines How New Zealand compares with









- Slides: 11

Reclassification of Medicines How New Zealand compares with other countries – March 2012

Why did we do this analysis? n To investigate concerns of risk-averse behaviour by Medsafe in switching classification status of medicines n Analysis conducted in December 2011

Sources of Data n The Association of the European Self- Medication Industry (AESGP) – January 2011 n Selected Regulatory Authorities schedules of medicines – December 2011 n World Self-Medication Industry (WSMI) website n No changes to the source data as at 28 March 2012

What countries were compared? n Countries with established and comparable regulatory regimes n Countries selected for this analysis: n EU countries: n n Belgium, UK, Portugal, Finland, Sweden, Denmark, France, the Netherlands, Ireland, Italy, Austria, Germany, Spain, Greece Specific Non-EU countries: n Australia, the USA, Canada, Switzerland, Norway, Singapore, Japan

Medicines reviewed n Ingredients were selected by the AEGSP and published in their tables n available at www. wsmi. org/otc. htm n Presumably these are medicines of interest to the AEGSP and WSMI n For this analysis, “Restricted Medicine” category in NZ has been grouped with prescription medicines n Ingredients not registered in NZ have been excluded from the analysis

Findings – OTC in NZ n 155 ingredients listed by the AESGP are OTC in New Zealand 131 of the 155 OTC medicines in New Zealand are prescription medicines in one or more other countries n Only 24 are OTC in all countries n n Most comparable country to NZ is Australia, with only 14 of these ingredients classified as Prescription medicines

Findings – prescription in NZ n 48 ingredients listed by the AESGP are Prescription status in New Zealand None are OTC in all 21 comparable countries n 7 are also prescription status in all 21 comparable countries n n Most switched country is the UK where 18 of the 48 are available OTC n Australia has 6 of the 48 prescription medicines in NZ available OTC

Conclusions n Amongst comparable countries, New Zealand leads in having the largest number of medicines available OTC 14 more than the next least restrictive country (Australia) n 68 more than the most restrictive (the USA) n n Concerns of risk-averse behaviour are not upheld by the evidence

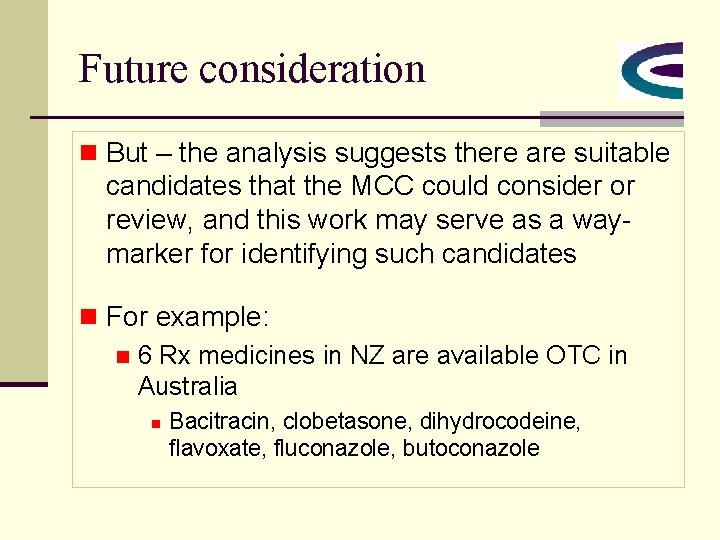
Future consideration n But – the analysis suggests there are suitable candidates that the MCC could consider or review, and this work may serve as a waymarker for identifying such candidates n For example: n 6 Rx medicines in NZ are available OTC in Australia n Bacitracin, clobetasone, dihydrocodeine, flavoxate, fluconazole, butoconazole

Examples n 14 OTC medicines in NZ are Rx in Australia n n Why? 18 Rx medicines in NZ are available OTC in the UK n Clemastine, codeine, pramocaine, domperidone, adenosine, oxybuprocaine, acrivastine, clobetasone, dihydrocodeine, mebeverine, fluconazole, sumatriptan, tranexamic acid, flunisolide, hydroxyzine, tamsulosin, azithromycin, simvastatin

n Thank you
 Figwort reclassification
Figwort reclassification Slidetodoc.com
Slidetodoc.com New zealand population
New zealand population Lesson 1 an introduction to oceania
Lesson 1 an introduction to oceania Saurabh paranjape new zealand
Saurabh paranjape new zealand Acn new zealand
Acn new zealand New zealand main language
New zealand main language Which is the capital of new zealand
Which is the capital of new zealand Internal medicine society of australia and new zealand
Internal medicine society of australia and new zealand New zealand disability strategy
New zealand disability strategy New zealand holiday 2016
New zealand holiday 2016 New zealand health strategy 2016
New zealand health strategy 2016