Recent Ongoing Studies in Wet ARMD Ahad Sedaghat













- Slides: 25

Recent & Ongoing Studies in Wet ARMD Ahad Sedaghat M. D. Rasool Akram Hospital ( IUMS )

• ARMD is the leading cause of visual impairment and blindness in the elderly in developed countries • anti-VEGF significantly improved treatment of wet-AMD. • approximately 40% of patients obtain full benefit from anti-VEGF therapy. • Anti-VEGF therapy also requires monthly or bimonthly injections • Therefore, it is required to develop options that have the potential not only to reduce patient visits and injections, but also to improve visual outcomes.

• Neovascularization or angiogenesis involves formation of new blood vessels from mature blood vessels. This is a multi-step process involving proliferation and migration of endothelial cells and pericytes. • Neovascularization is orchestrated by a variety of growth factors including : • vascular endothelial growth factor (VEGF), • basic-fibroblast growth factor (b-FGF) • platelet derived growth factor-B (PDGF-B)

• Although intravitreal injections of anti-VEGF therapy have significant therapeutic benefit, in some patients visual acuity does not improve or stabilize and some patients become refractory to therapy over time. • Dual targeting of VEGF and PDGF-B signaling pathways have shown synergistic inhibition of angiogenesis in tumor and ocular neovascularization models

PDGF Inhibitors • PDGF stimulates angiogenesis, as well as pericyte maturation • Inhibition of PDGF increases endothelial cell sensitivity to anti-VEGF agents. • E 10030 (Fovista®) is an aptamer directed against PDGF and is currently enrolling in parallel phase 3 clinical trials in combination with anti-VEGF injections. • E 10030 decreases pericyte density in neovascular vessels and therefore improves the effect of anti-VEGF agents

• A phase 2 clinical trial evaluated the efficacy and safety of E 10030 administered in combination with an anti-VEGF agent for the treatment of patients newly diagnosed wet AMD. • E 10030 (0. 3 mg or 1. 5 mg) administered in combination with ranibizumab injection (0. 5 mg) demonstrated statistically significant improvement compared to ranibizumab monotherapy based on the primary endpoint of mean change in visual acuity from baseline at 24 weeks. • E 10030 exhibited a favorable safety profile and no serious adverse effects were observed for E 10030 combination therapy.

Squalamine Eye Drops (OHR-102) • Squalamine is an anti-angiogenic small molecule with a novel intracellular mechanism of action, which counteracts multiple growth factors and pathways implicated in the angiogenic process, including VEGF, PDGF, and b-FGF. • A Phase II randomized, double masked, placebo-controlled study to evaluate the efficacy and safety of Squalamine eye drops for the treatment of wet AMD is ongoing and has completed enrollment. Interim data demonstrated benefit in visual function versus placebo across multiple standard parameters.

Take-Home Message: • Combination treatment with squalamine 0. 2% and ranibizumab PRN resulted in improved visual function in patients with neovascular ARMD compared with treatment with ranibizumab alone. • particularly in eyes with classic-containing CNV lesions.

Anti-Immune or Anti-Inflammatory Pathways • Newman et al. reported cell-mediated immune responses as the central feature of all AMD phenotypes. Therefore, addressing the role of immune response in the pathogenesis of ocular neovascularization may be a promising avenue for identifying targets for AMD treatments. • Mammalian target of rapamycin (m. TOR) inhibitor • Anti-tumor necrosis factor alpha (TNF-a) antibody • Complement component 3 (C 3) inhibitor

Sirolimus (previously known as rapamycin) Mammalian target of rapamycin (m. TOR) inhibitor Originally developed as a macrolide antifungal agent but was found to possess potent immunosuppressive and antiproliferative properties. Sirolimus blocks the T-lymphocyte activation and smooth muscle and endothelial cell proliferation. (interleukins IL-2, IL-4, and IL-15) • Sirolimus is used to prevent rejection after organ transplantation, particularly after renal transplantation.

• Sirolimus markedly inhibits VEGF and inhibits hypoxia-inducible factor-1α, a major upstream regulator of VEGF. • In a murine AMD model, systemic administration of sirolimus inhibited both choroidal and retinal neovascularization.

• Results from earlier phase 1 and phase 2 studies showed that Sirolimus was safe and well tolerated. • There was no evidence of increased IOP, inflammatory response to treatment, or indication of progression of cataracts. • Patients showed improvements in visual acuity and improvements in retinal thickness.

TNF-α inhibitors ( infliximab ) • TNF-α is a key molecule that plays a central role in inflammation, apoptosis, and immune system. • The anti-TNF-α monoclonal antibody infliximab is used for various inflammatory diseases, such as rheumatoid arthritis. • Regression of CNV and improvement of visual acuity were reported in three wet AMD patients treated with intravenous infliximab for inflammatory arthritis.

• Intravitreally administered infliximab is safely increased up to 2 mg in the rabbit eye. • Intravitreal infliximab was administered to three patients with wet AMD, resulting in improved visual acuity and central foveal thickness on OCT. • A phase 1 study is now underway and is evaluating intravitreal infliximab for wet AMD, as well as for refractory DME.

C 3 inhibitors • C 3, is a protein of the immune system. It plays a central role in the complement system and contributes to innate immunity. • C 3 is detected in the vicinity of the drusen. • Genetic studies revealed highly significant statistical associations between AMD and variants of C 3 gene. • C 3 induces VEGF expression in vivo and in vitro, and C 3 gene polymorphism increases the risk of AMD.

POT-4 ( C 3 inhibitor) • POT-4 is a synthetic peptide of 13 amino acids and “gel-like” product when injected into the vitreous. • The effect is long-lasting (3 to 6 months). • A phase 1 study will provide safety and tolerability information on POT-4 injected into the eyes of patients with wet AMD successfully completes phase 1 clinical trial. • POT-4 also demonstrated therapeutic efficacy in a few end-stage wet AMD patients.

Axitinib • Axitinib, a small molecule multi-receptor tyrosine kinase inhibitor used for the treatment of advanced renal cell carcinoma, is taken orally and inhibits VEGF activity by blocking VEGF receptors. • Axitinib also has the advantage of blocking PDGF receptors which play a role in neovascularization. • Axitinib inhibited neovascularization better than anti-VEGF and/or anti-PDGF in the in vitro models.

• one study showed that continuous administration of axitinib at a low dose (0. 875 mg/day) for 7 days significantly inhibited vascular leakage and neovascularization in rat CNV model. • It has been shown that co-administration of axitinib with an anti-VEGF increased the anti- angiogenesis effect of the anti-VEGF.

MTX (Methotrexate) • MTX is an antifolate drug and is used in high doses to treat cancer. Primary intraocular lymphoma is treated with intravitreal MTX • Kurup et al. reported that two wet AMD patients were refractory to anti-VEGF treatment but showed some improvement by intravitreal MTX injection (400 mg). One patient showed improved visual acuity, and both patients showed decreased serous retinal detachment and decreased perifoveal leakage 2 weeks after injection. • large-scale and long-term clinical trials are necessary to evaluate the effectiveness of intravitreal MTX.

The First Stem Cell Clinical Trial for Wet Macular Degeneration Is Underway in London • A pioneering clinical trial of a new treatment derived from embryonic stem cells for people with wet AMD has been initiated at Moorfields Eye Hospital in London • The ongoing clinical trial is investigating the safety and effectiveness of transplanting RPE cells derived from stem cells to treat people with sudden severe visual loss from wet AMD. • the trial will recruit 10 patients over a period of 18 months. Each patient will be followed for a year to assess the safety and stability of the cells and the degree to which their vision is restored. • The first surgery was performed in August 2015 and there have been no reported complications to date. The surgical team hopes to determine the outcome, in terms of initial visual recovery, by early December 2016.

Radiation therapy • Radiation is used to selectively and irreversibly damage the DNA of target cells which prevents further replication. • Cells that are rapidly dividing, or of abnormal morphology, undergo apoptosis following radiation therapy, whereas nondividing cells are better able to repair the damage and remain structurally intact. • Treatment with radiation can be divided into two categories: Brachytherapy: known as internal radiotherapy Teletherapy: uses an external source

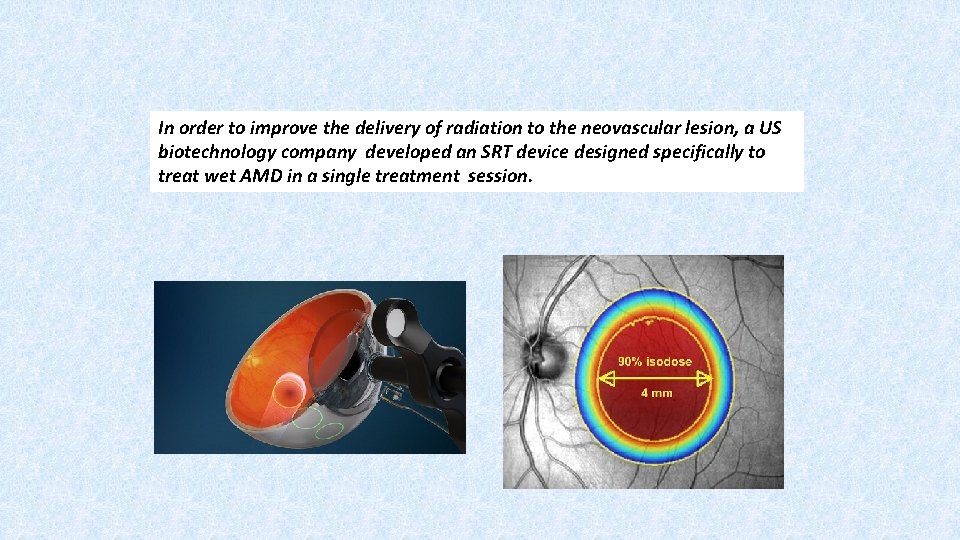
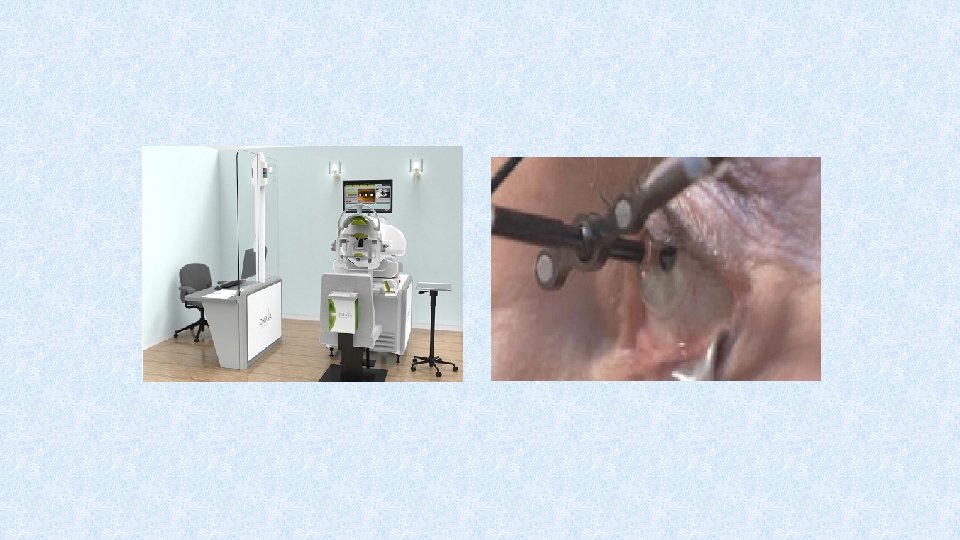
In order to improve the delivery of radiation to the neovascular lesion, a US biotechnology company developed an SRT device designed specifically to treat wet AMD in a single treatment session.


CABERNET – Strontium-90 Beta Radiation Implant Trial • Previous research had led to the hope that radiation treatment could be used with Lucentis to reduce the number of injections that were needed. • A tiny source of radiation is placed inside the eye near the macula, held there for about 4 minutes and then removed. • The radiation destroys the abnormal blood vessels and prevents the growth of blood vessels to stop the progression of wet macular degeneration vision loss.
In a previous study, 34 people with wet macular degeneration received a single treatment of epiretinal brachytherapy Patients received the radiation and two injections of Avastin® - one dose just before the radiation or at the time of radiation delivery and an additional injection of Avastin® one month later 76 percent of the patients in the study did not require additional injections of Avastin® throughout the year. • After 12 months, 94 percent of patients lost fewer than 15 letters, 39 percent gained 15 or more letters, and 12 percent gained 30 or more letters.