Recent Atomic Models Max Planck 1900 Proposed that



![Shorthand Configuration Review Element symbol Electron configuration Ca [Ar] 4 s 2 V [Ar] Shorthand Configuration Review Element symbol Electron configuration Ca [Ar] 4 s 2 V [Ar]](https://slidetodoc.com/presentation_image_h2/79701fc1a41b81beee5b948b04f34f91/image-41.jpg)
![lithium atom, Li 3 p +, 3 e – [He] 2 s 1 sodium lithium atom, Li 3 p +, 3 e – [He] 2 s 1 sodium](https://slidetodoc.com/presentation_image_h2/79701fc1a41b81beee5b948b04f34f91/image-48.jpg)
- Slides: 51

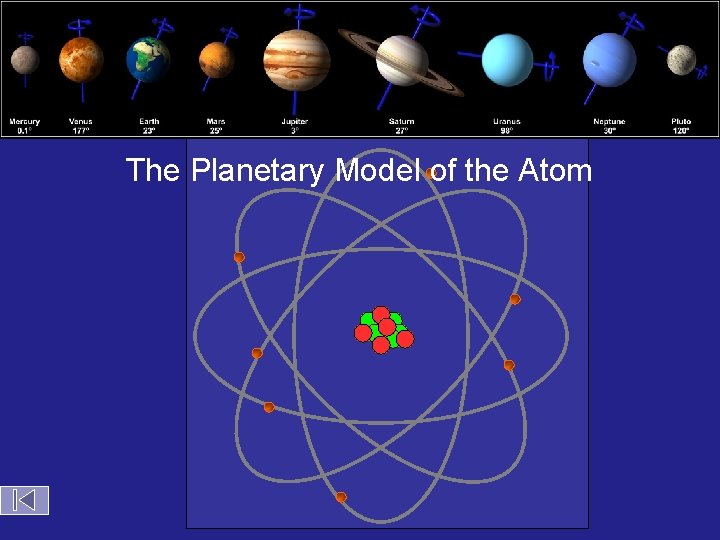
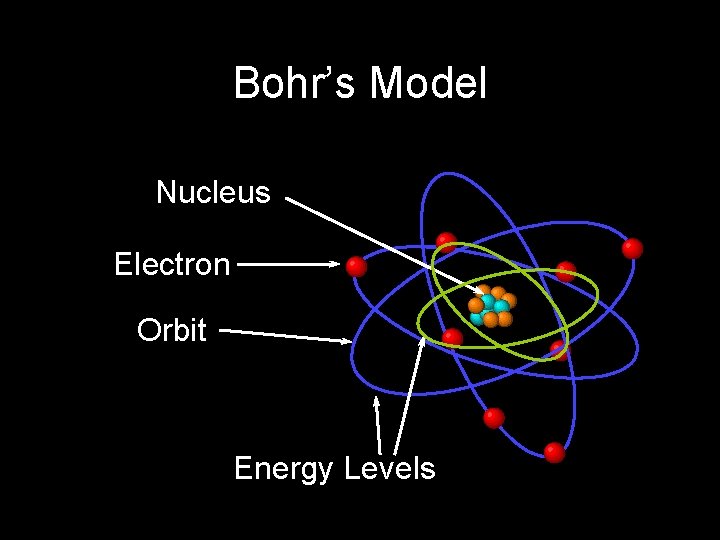
Recent Atomic Models Max Planck (1900): Proposed that amounts of energy are quantized only certain E values are allowed Niels Bohr (1913): e– can possess only certain amounts of energy, and can therefore be only certain distances from nucleus. planetary (Bohr) model e– found here N e– never found here

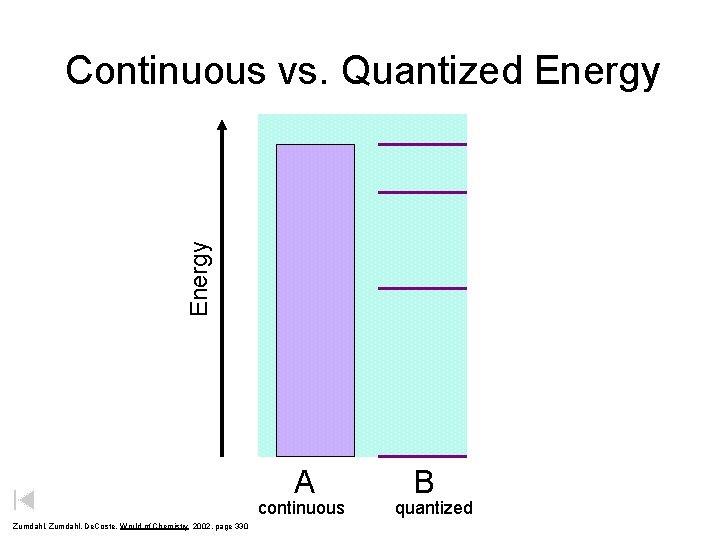
Energy Continuous vs. Quantized Energy A continuous Zumdahl, De. Coste, World of Chemistry 2002, page 330 B quantized

Bohr Atom The Planetary Model of the Atom

Bohr’s Model Nucleus Electron Orbit Energy Levels

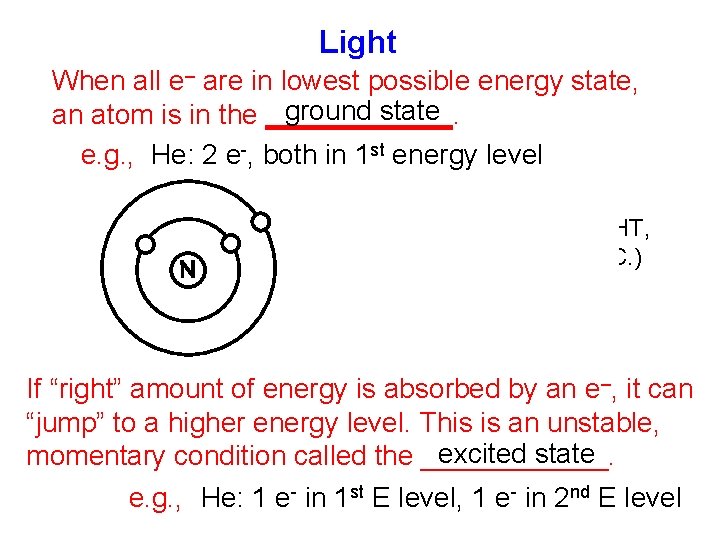
Light When all e– are in lowest possible energy state, ground state an atom is in the ______. e. g. , He: 2 e-, both in 1 st energy level ENERGY (HEAT, LIGHT, ELEC. , ETC. ) If “right” amount of energy is absorbed by an e–, it can “jump” to a higher energy level. This is an unstable, excited state momentary condition called the ______. e. g. , He: 1 e- in 1 st E level, 1 e- in 2 nd E level

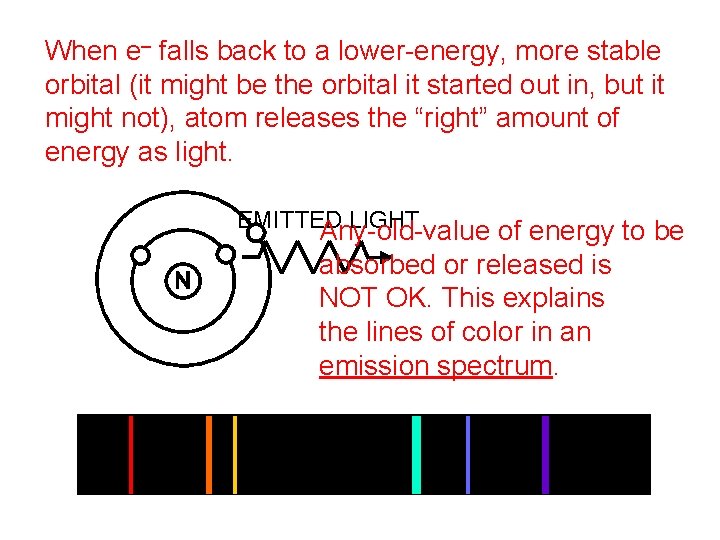
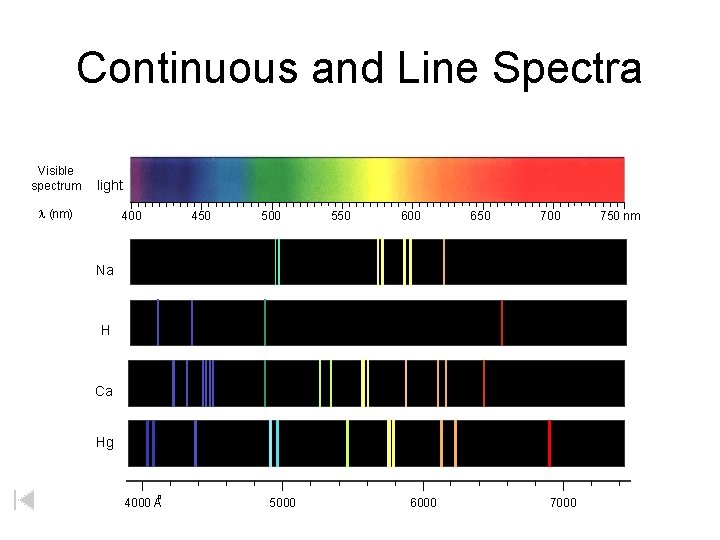
When e– falls back to a lower-energy, more stable orbital (it might be the orbital it started out in, but it might not), atom releases the “right” amount of energy as light. EMITTED LIGHT Any-old-value of energy to be absorbed or released is NOT OK. This explains the lines of color in an emission spectrum.

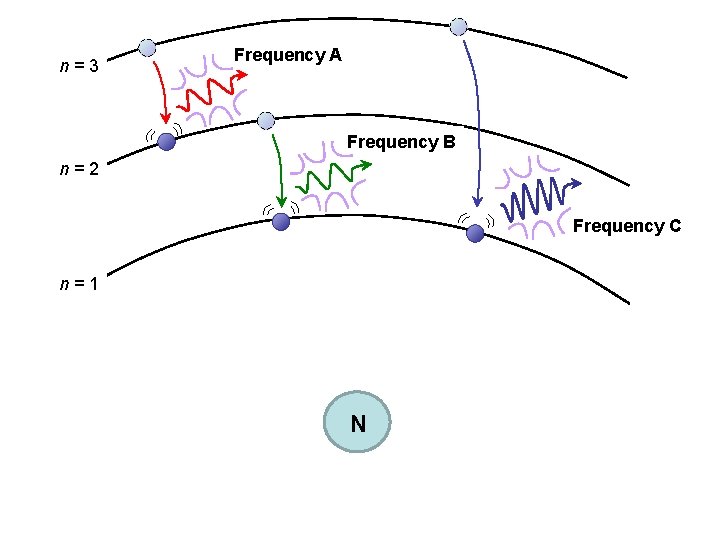
n=3 Frequency A Frequency B n=2 Frequency C n=1 N

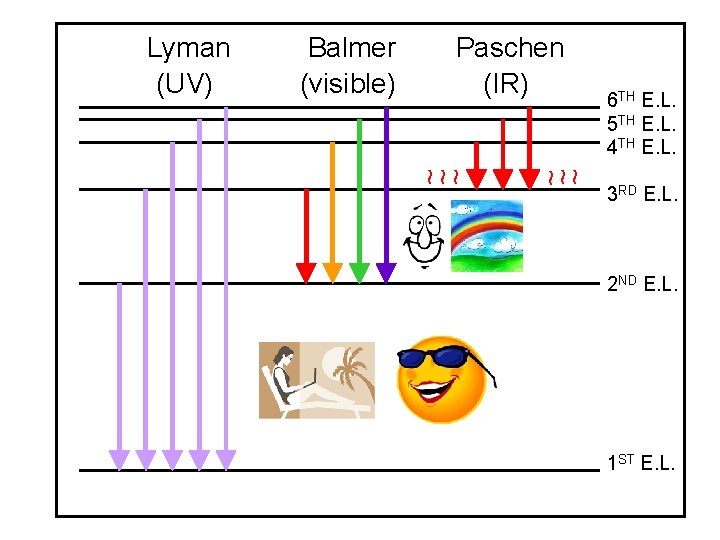
Lyman (UV) Balmer (visible) Paschen (IR) 6 TH E. L. 5 TH E. L. 4 TH E. L. ~ ~ ~ 3 RD E. L. 2 ND E. L. 1 ST E. L.

Continuous and Line Spectra Visible spectrum light l (nm) 400 450 500 550 600 650 700 Na H Ca Hg o 4000 A 5000 6000 750 nm

Flame Emission Spectra Photographs of flame tests of burning wooden splints soaked in different salts. methane gas wooden splint sodium ion calcium ion copper ion strontium ion Include link to web page http: //www. unit 5. org/christjs/flame%20 tests. htm

Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.

Fireworks Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.

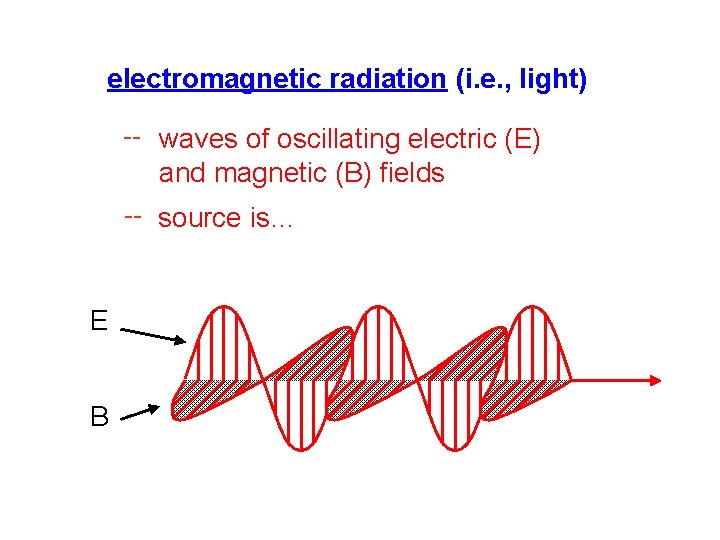
electromagnetic radiation (i. e. , light) -- waves of oscillating electric (E) and magnetic (B) fields -- source is… vibrating electric charges E B

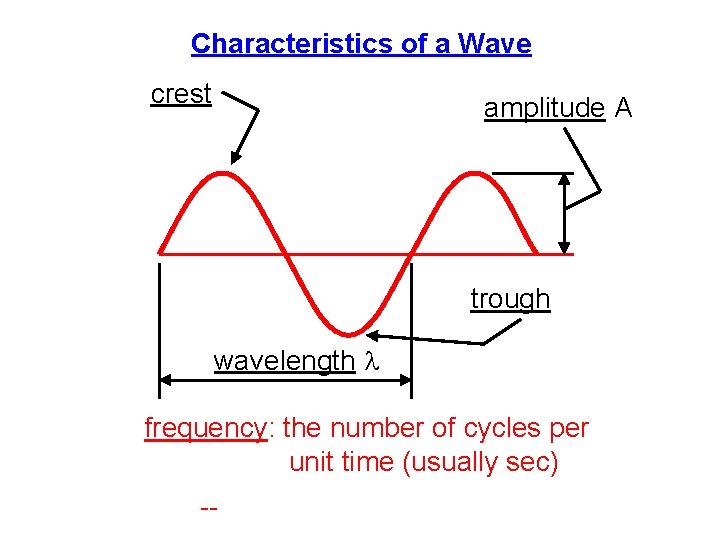
Characteristics of a Wave crest amplitude A trough wavelength l frequency: the number of cycles per unit time (usually sec) -- unit is Hz, or s– 1 or 1/s

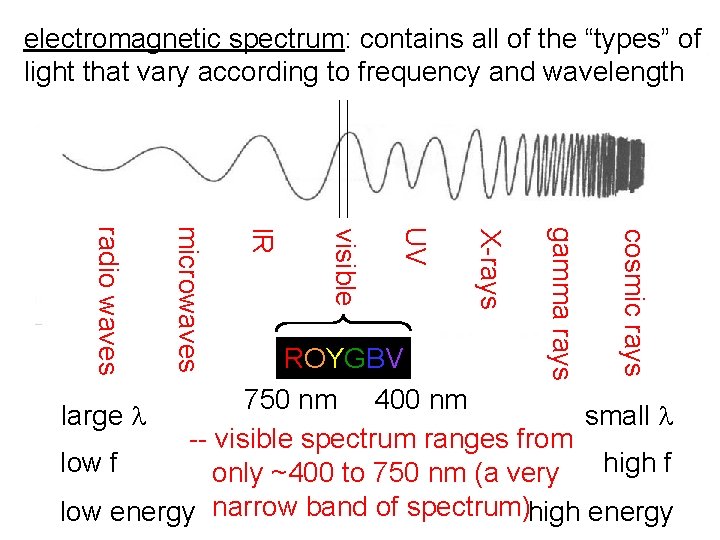
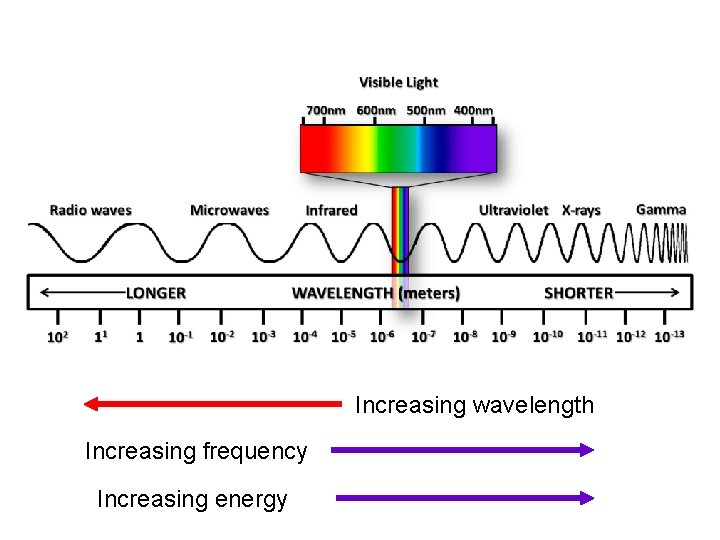
electromagnetic spectrum: contains all of the “types” of light that vary according to frequency and wavelength cosmic rays gamma rays X-rays UV visible IR microwaves radio waves ROYGBV 750 nm 400 nm large l small l -- visible spectrum ranges from low f high f only ~400 to 750 nm (a very low energy narrow band of spectrum)high energy

Increasing wavelength Increasing frequency Increasing energy

Some light humor…

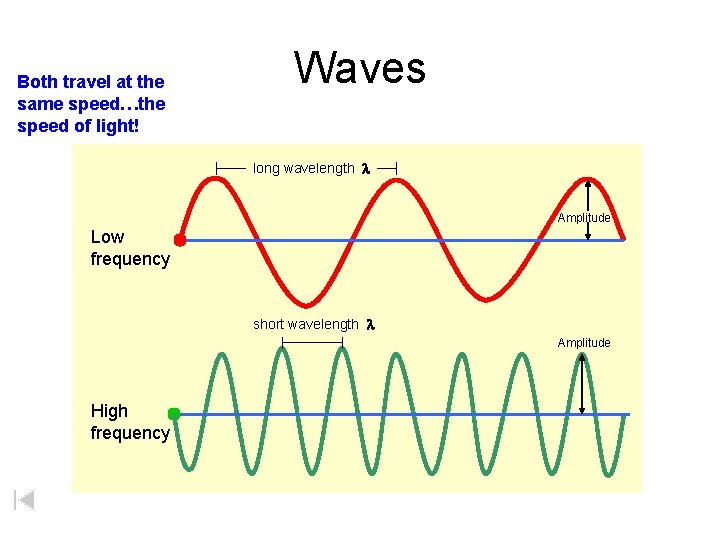
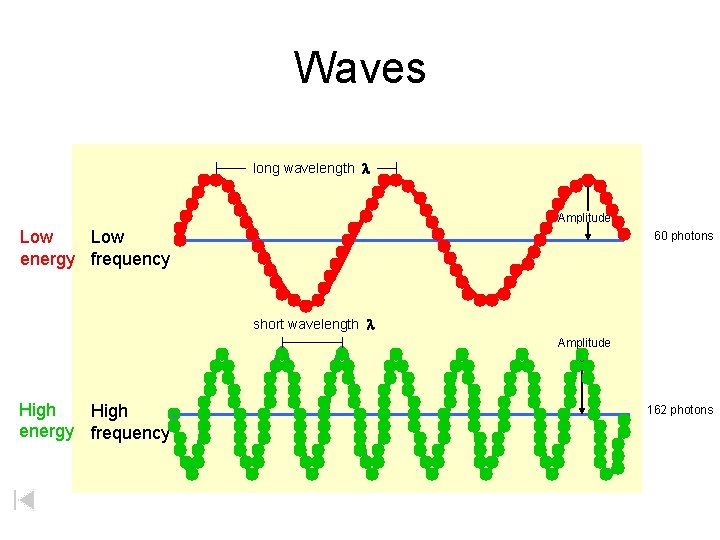
Both travel at the same speed…the speed of light! Waves long wavelength l Amplitude Low frequency short wavelength l Amplitude High frequency

Waves long wavelength l Amplitude Low energy frequency 60 photons short wavelength l Amplitude High energy frequency 162 photons

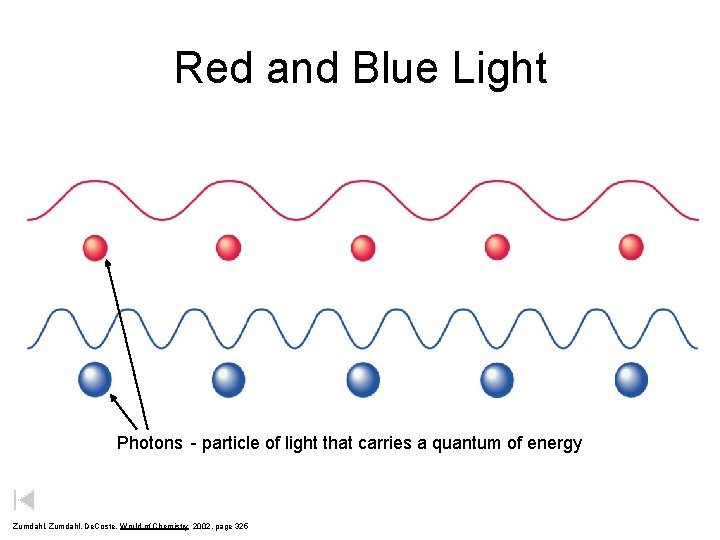
Red and Blue Light Photons - particle of light that carries a quantum of energy Zumdahl, De. Coste, World of Chemistry 2002, page 325

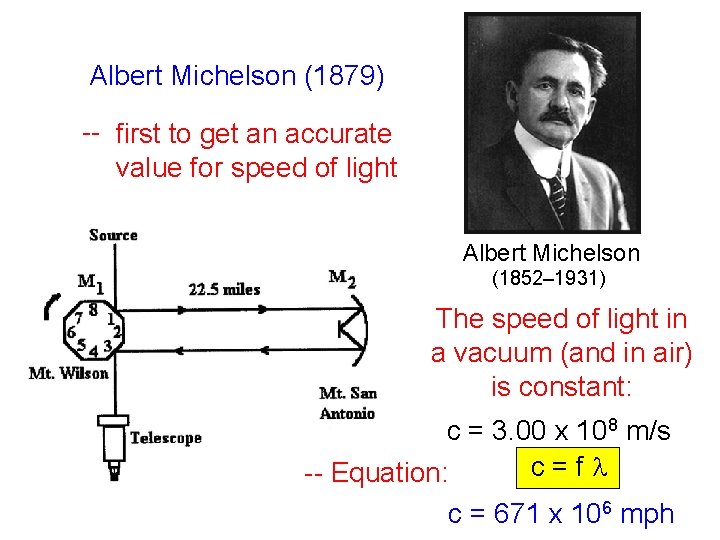
Albert Michelson (1879) -- first to get an accurate value for speed of light Albert Michelson (1852– 1931) The speed of light in a vacuum (and in air) is constant: c = 3. 00 x 108 m/s c=fl -- Equation: c = 671 x 106 mph

In 1900, Max Planck assumed that energy can be absorbed or released only in certain discrete amounts, which he called quanta. Later, Albert Einstein dubbed a light “particle” that carried a quantum of energy a photon. -- Equation: Max Planck (1858– 1947) E=hf E = energy, in J h = Planck’s constant = 6. 63 x 10– 34 J∙s (i. e. , J/Hz) Albert Einstein (1879– 1955)

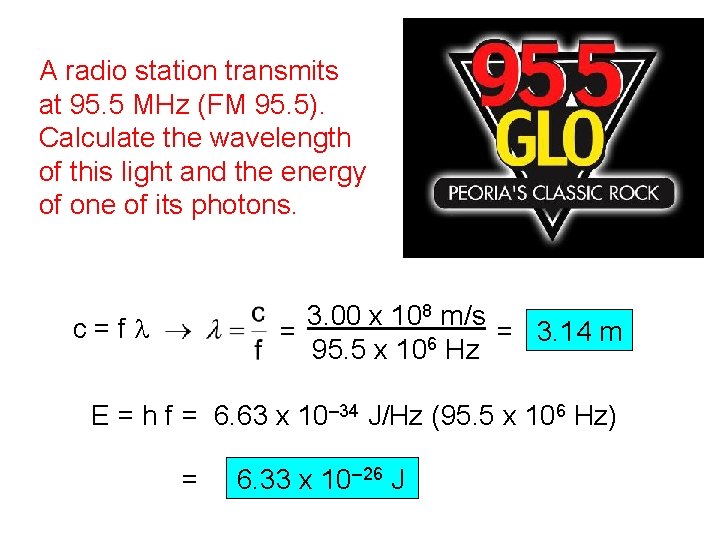
A radio station transmits at 95. 5 MHz (FM 95. 5). Calculate the wavelength of this light and the energy of one of its photons. 3. 00 x 108 m/s = 3. 14 m = 6 95. 5 x 10 Hz c=fl E = h f = 6. 63 x 10– 34 J/Hz (95. 5 x 106 Hz) = 6. 33 x 10– 26 J

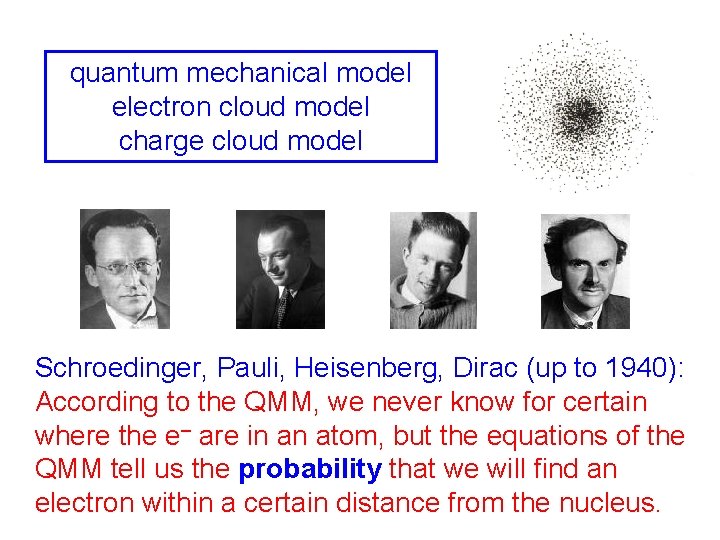
quantum mechanical model electron cloud model charge cloud model Schroedinger, Pauli, Heisenberg, Dirac (up to 1940): According to the QMM, we never know for certain where the e– are in an atom, but the equations of the QMM tell us the probability that we will find an electron within a certain distance from the nucleus.

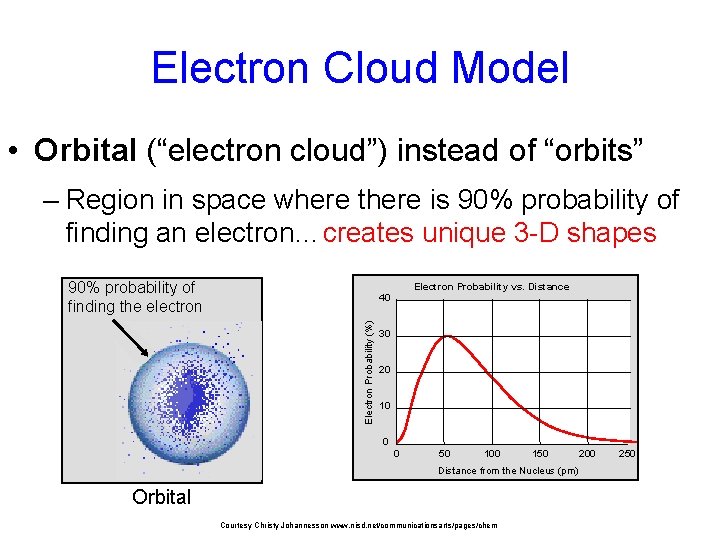
Electron Cloud Model • Orbital (“electron cloud”) instead of “orbits” – Region in space where there is 90% probability of finding an electron…creates unique 3 -D shapes 90% probability of finding the electron Electron Probability vs. Distance Electron Probability (%) 40 30 20 10 0 0 50 100 150 Distance from the Nucleus (pm) Orbital Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem 200 250

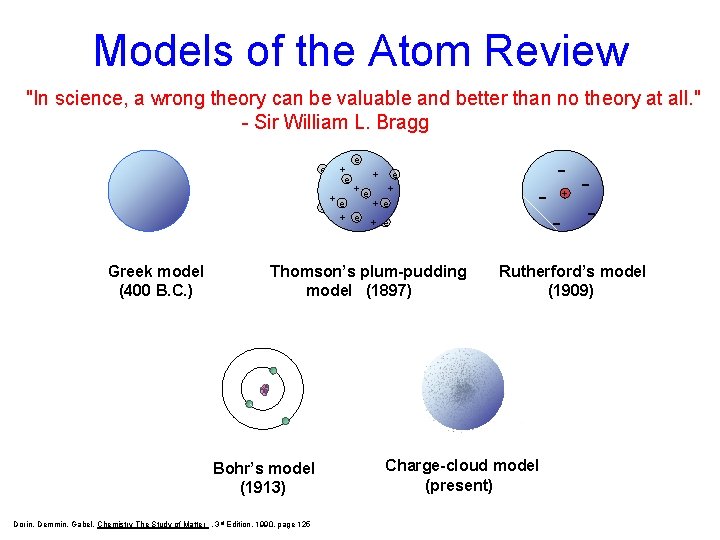
Models of the Atom Review "In science, a wrong theory can be valuable and better than no theory at all. " - Sir William L. Bragg e + e +e + e + e Dalton’s Greek model (400 (1803) B. C. ) Thomson’s plum-pudding model (1897) Bohr’s model (1913) Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 125 - - + Rutherford’s model (1909) Charge-cloud model (present)

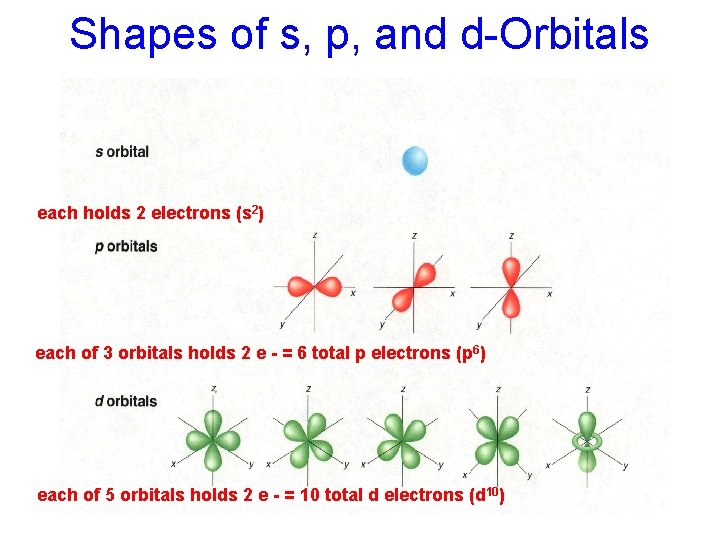
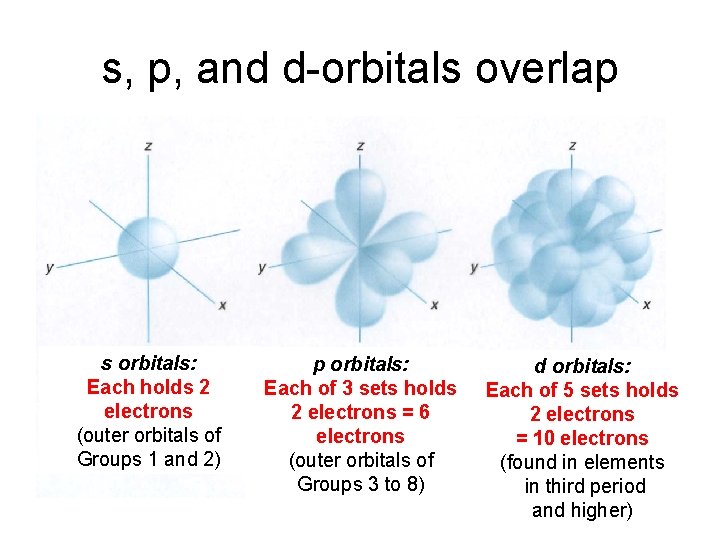
Shapes of s, p, and d-Orbitals each holds 2 electrons (s 2) each of 3 orbitals holds 2 e - = 6 total p electrons (p 6) each of 5 orbitals holds 2 e - = 10 total d electrons (d 10)

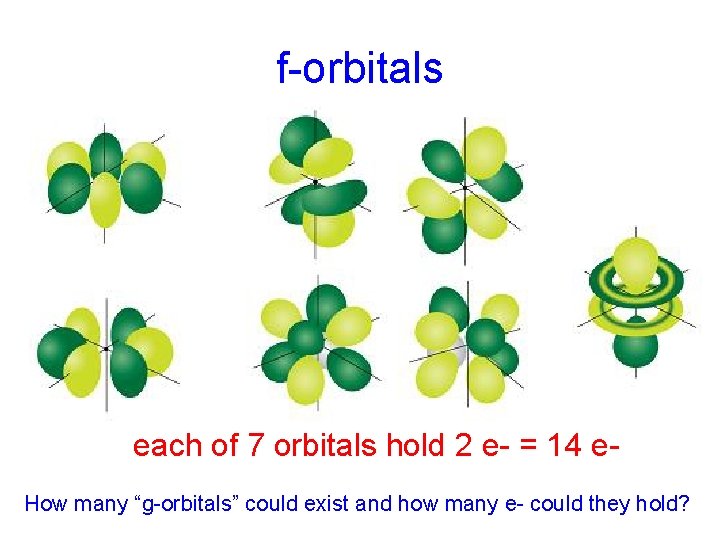
f-orbitals each of 7 orbitals hold 2 e- = 14 e. How many “g-orbitals” could exist and how many e- could they hold?

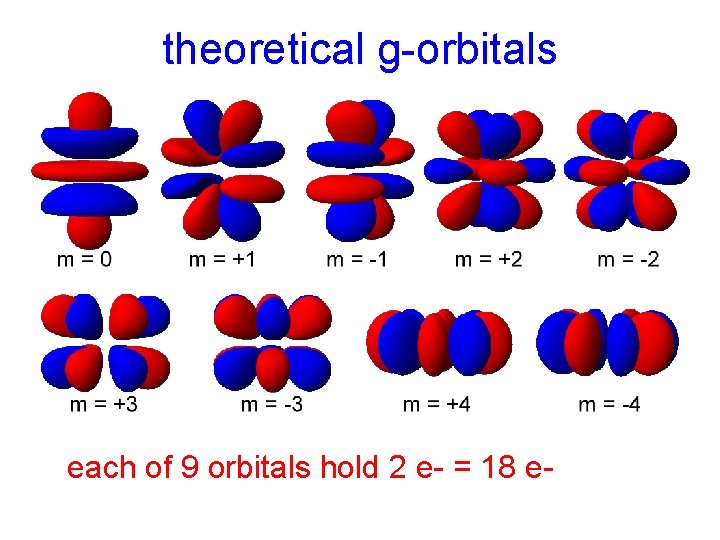
theoretical g-orbitals each of 9 orbitals hold 2 e- = 18 e-

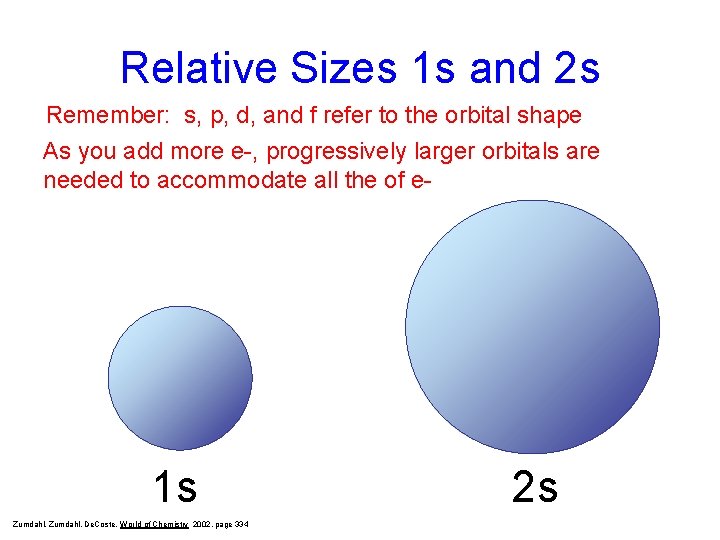
Relative Sizes 1 s and 2 s Remember: s, p, d, and f refer to the orbital shape As you add more e-, progressively larger orbitals are needed to accommodate all the of e- 1 s Zumdahl, De. Coste, World of Chemistry 2002, page 334 2 s

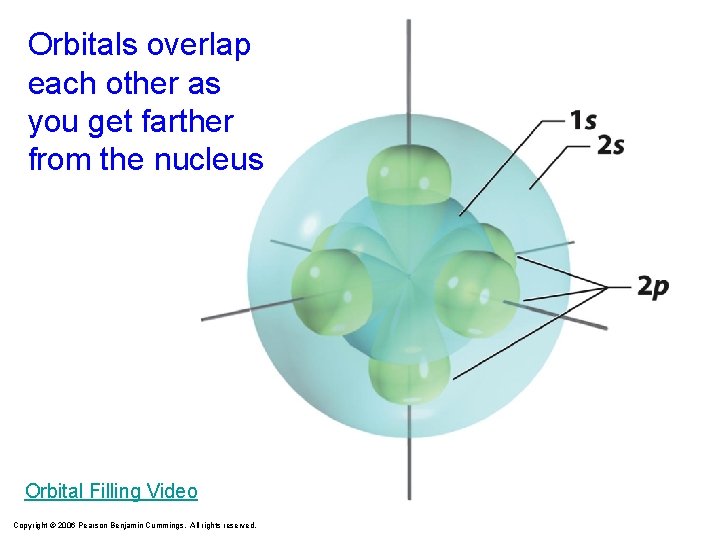
Orbitals overlap each other as you get farther from the nucleus Orbital Filling Video Copyright © 2006 Pearson Benjamin Cummings. All rights reserved.

s, p, and d-orbitals overlap s orbitals: Each holds 2 electrons (outer orbitals of Groups 1 and 2) p orbitals: Each of 3 sets holds 2 electrons = 6 electrons (outer orbitals of Groups 3 to 8) d orbitals: Each of 5 sets holds 2 electrons = 10 electrons (found in elements in third period and higher)

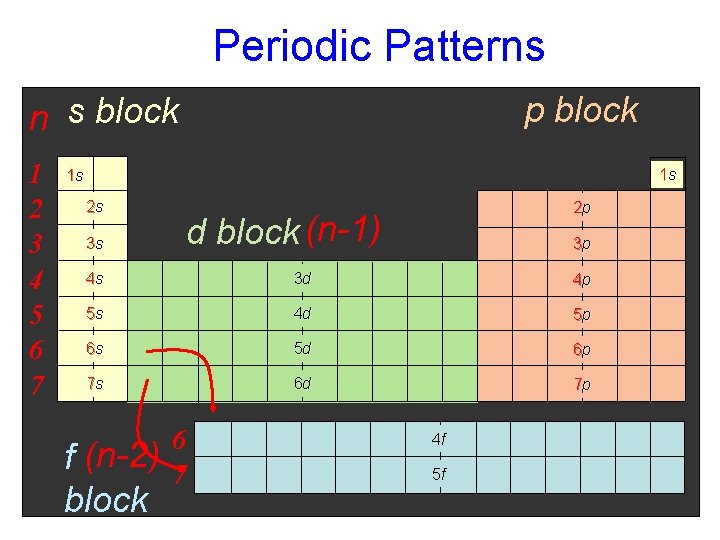
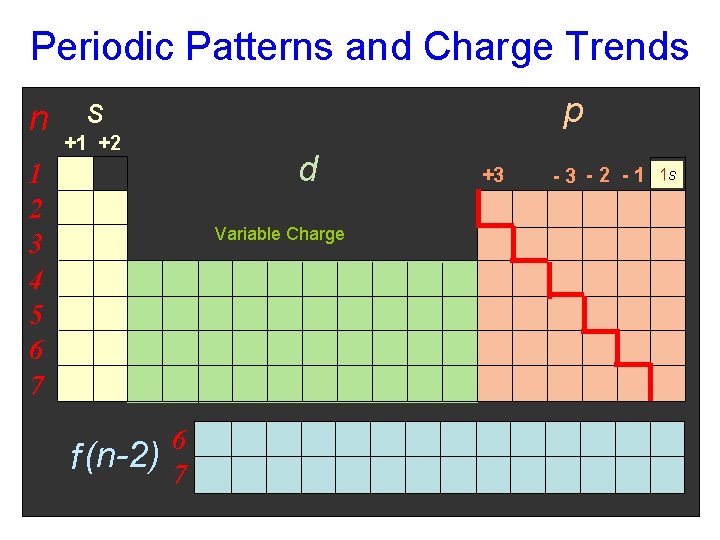
Periodic Patterns p block n s block 1 2 3 4 5 6 7 1 s 1 s 2 s 2 p d block (n-1) 3 s 3 p 4 s 3 d 4 p 5 s 4 d 5 p 6 s 5 d 6 p 7 s 6 d 7 p f (n-2) block 6 7 4 f 5 f

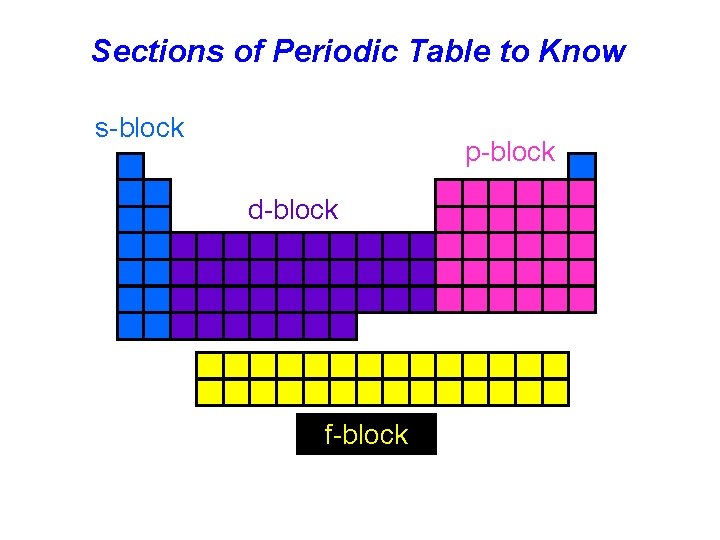
Sections of Periodic Table to Know s-block p-block d-block f-block

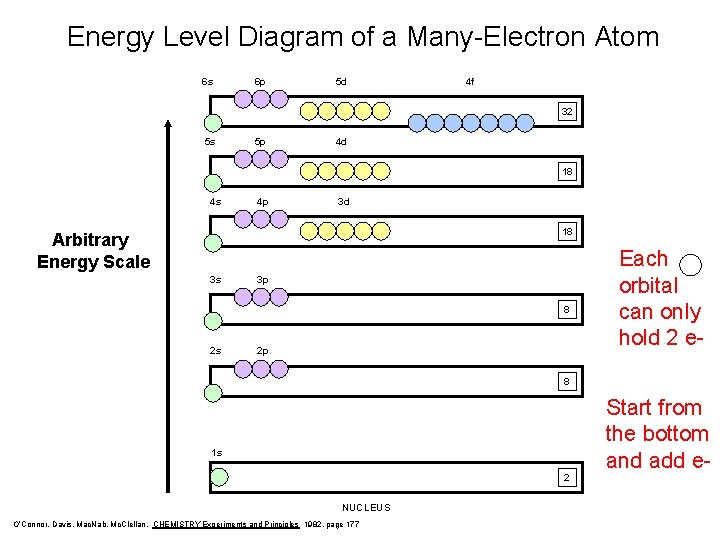
Energy Level Diagram of a Many-Electron Atom 6 s 6 p 5 d 4 f 32 5 s 5 p 4 d 18 4 s 4 p 3 d 18 Arbitrary Energy Scale 3 s 3 p 8 2 s 2 p Each orbital can only hold 2 e- 8 1 s 2 NUCLEUS O’Connor, Davis, Mac. Nab, Mc. Clellan, CHEMISTRY Experiments and Principles 1982, page 177 Start from the bottom and add e-

You don’t have to memorize the order…just start at the beginning and fill in e-…

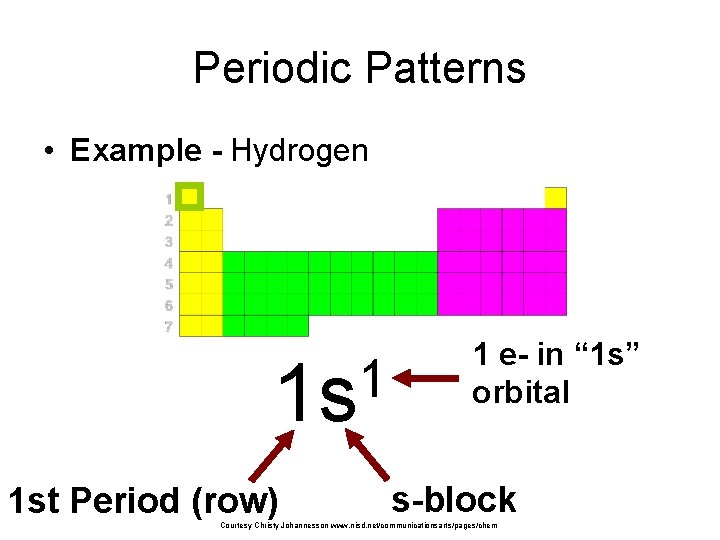
Periodic Patterns • Example - Hydrogen 1 1 s 1 st Period (row) 1 e- in “ 1 s” orbital s-block Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

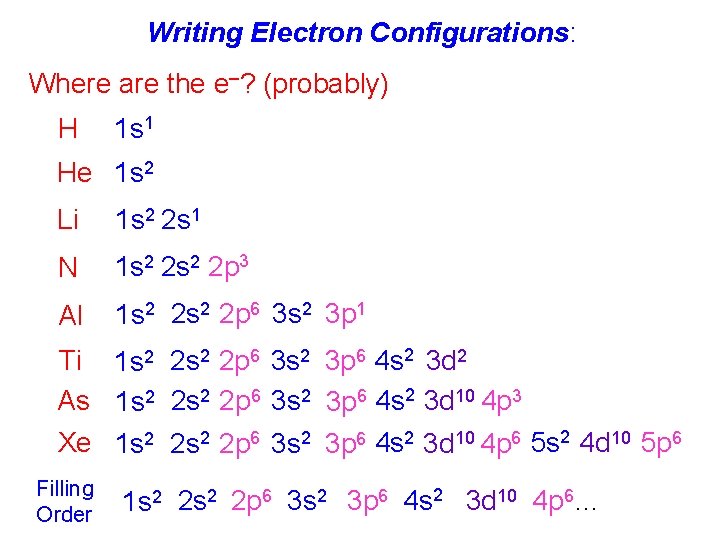
Writing Electron Configurations: Where are the e–? (probably) H 1 s 1 He 1 s 2 Li 1 s 2 2 s 1 N 1 s 2 2 p 3 Al 1 s 2 2 p 6 3 s 2 3 p 1 Ti 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 2 As 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 3 Xe 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6 Filling Order 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6…

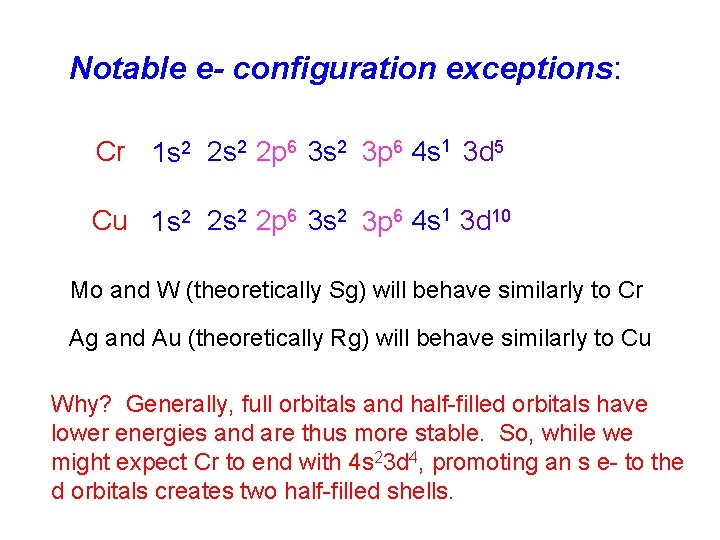
Notable e- configuration exceptions: Cr 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 5 Cu 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10 Mo and W (theoretically Sg) will behave similarly to Cr Ag and Au (theoretically Rg) will behave similarly to Cu Why? Generally, full orbitals and half-filled orbitals have lower energies and are thus more stable. So, while we might expect Cr to end with 4 s 23 d 4, promoting an s e- to the d orbitals creates two half-filled shells.

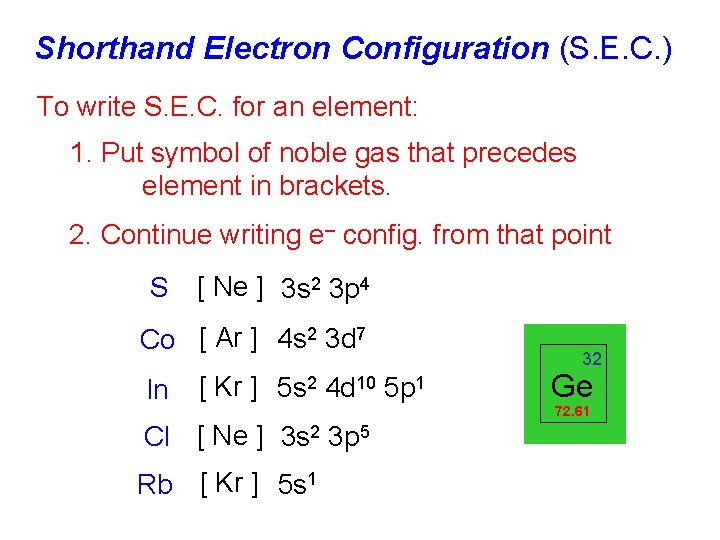
Shorthand Electron Configuration (S. E. C. ) To write S. E. C. for an element: 1. Put symbol of noble gas that precedes element in brackets. 2. Continue writing e– config. from that point S [ Ne ] 3 s 2 3 p 4 Co [ Ar ] 4 s 2 3 d 7 In [ Kr ] 5 s 2 4 d 10 5 p 1 Cl [ Ne ] 3 s 2 3 p 5 Rb [ Kr ] 5 s 1 32 Ge 72. 61
![Shorthand Configuration Review Element symbol Electron configuration Ca Ar 4 s 2 V Ar Shorthand Configuration Review Element symbol Electron configuration Ca [Ar] 4 s 2 V [Ar]](https://slidetodoc.com/presentation_image_h2/79701fc1a41b81beee5b948b04f34f91/image-41.jpg)
Shorthand Configuration Review Element symbol Electron configuration Ca [Ar] 4 s 2 V [Ar] 4 s 2 3 d 3 F [He] 2 s 2 2 p 5 Ag [Kr] 5 s 1 4 d 10 I [Kr] 5 s 2 4 d 10 5 p 5 Xe [Kr] 5 s 2 4 d 10 5 p 6 or [Xe] Fe 22 p 64 s [He] 2 s[Ar] 3 s 223 d 3 p 664 s 23 d 6 Sg [Rn] 7 s 2 5 f 14 6 d 4

Three Principles about Electrons 4 s 2 3 p 6 3 s 2 aufbau Principle: e– will fill the lowest-energy orbital available Hund’s Rule: 3 d 10… 2 p 6 2 s 2 1 s 2 for equal-energy orbitals (p, d) each must have one e– before any take a second Friedrich Hund Pauli Exclusion Principle: two e– in same orbital have different spins Wolfgang Pauli

Orbital Diagrams …show spins of e– and orbital location O 1 s 22 p 4 1 s P 2 s 2 p 3 s 3 p 1 s 22 p 63 s 23 p 3 1 s 2 s 2 p

The Importance of Electrons orbitals: regions of space where an e– may be found In a generic e– config (e. g. , 1 s 2 2 p 6 3 s 2 3 p 6…): coefficient # of energy level superscript # of e– in those orbitals In general, as energy level # increases, e–… HAVE MORE ENERGY AND ARE FURTHER FROM NUCLEUS

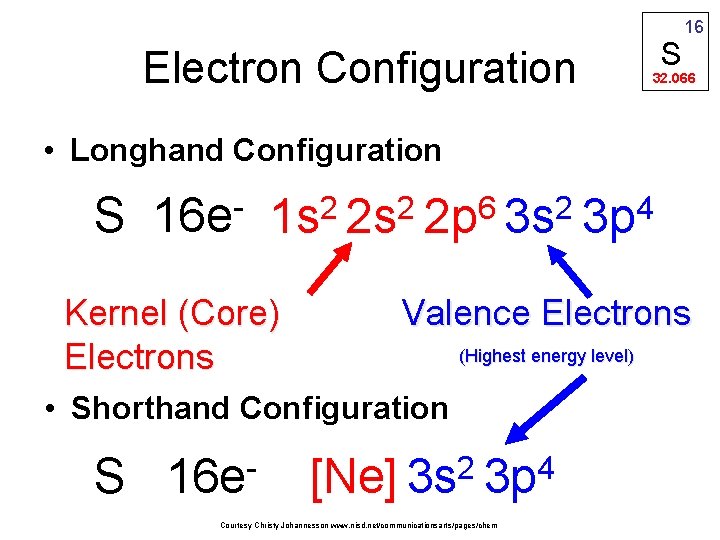
S Electron Configuration 16 32. 066 • Longhand Configuration S 16 e 6 2 2 2 1 s 2 s 2 p 3 s Kernel (Core) Electrons Valence Electrons (Highest energy level) • Shorthand Configuration S 16 e 4 3 p 2 4 [Ne] 3 s 3 p Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

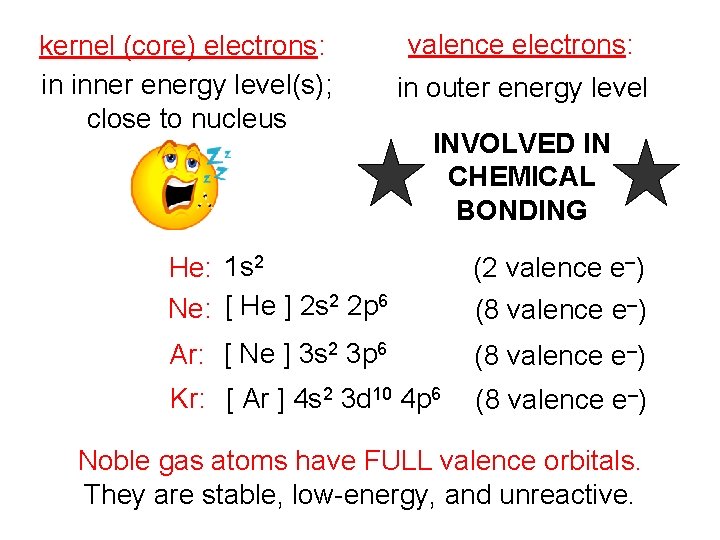
kernel (core) electrons: in inner energy level(s); close to nucleus valence electrons: in outer energy level INVOLVED IN CHEMICAL BONDING He: 1 s 2 Ne: [ He ] 2 s 2 2 p 6 (2 valence e–) Ar: [ Ne ] 3 s 2 3 p 6 (8 valence e–) Kr: [ Ar ] 4 s 2 3 d 10 4 p 6 (8 valence e–) Noble gas atoms have FULL valence orbitals. They are stable, low-energy, and unreactive.

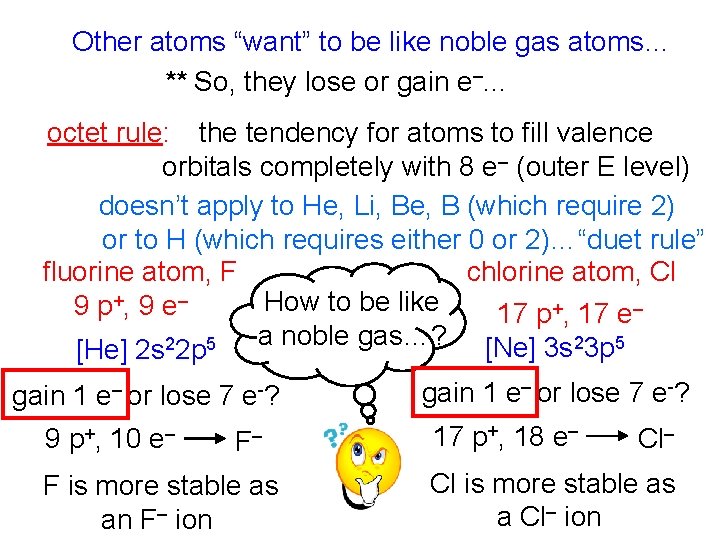
Other atoms “want” to be like noble gas atoms… ** So, they lose or gain e–. . . octet rule: the tendency for atoms to fill valence orbitals completely with 8 e– (outer E level) doesn’t apply to He, Li, Be, B (which require 2) or to H (which requires either 0 or 2)…“duet rule” chlorine atom, Cl fluorine atom, F How to be like 9 p +, 9 e – 17 p+, 17 e– [He] 2 s 22 p 5 a noble gas…? [Ne] 3 s 23 p 5 gain 1 e– or lose 7 e-? 9 p+, 10 e– F– F is more stable as an F– ion gain 1 e– or lose 7 e-? 17 p+, 18 e– Cl is more stable as a Cl– ion
![lithium atom Li 3 p 3 e He 2 s 1 sodium lithium atom, Li 3 p +, 3 e – [He] 2 s 1 sodium](https://slidetodoc.com/presentation_image_h2/79701fc1a41b81beee5b948b04f34f91/image-48.jpg)
lithium atom, Li 3 p +, 3 e – [He] 2 s 1 sodium atom, Na How to be like a noble gas…? lose 1 e– or gain 7 e-? 3 p +, 2 e – Li+ Li is more stable as the Li+ ion. 11 p+, 11 e– [Ne] 3 s 1 lose 1 e– or gain 7 e-? 11 p+, 10 e– Na+ Na is more stable as Na+ ion

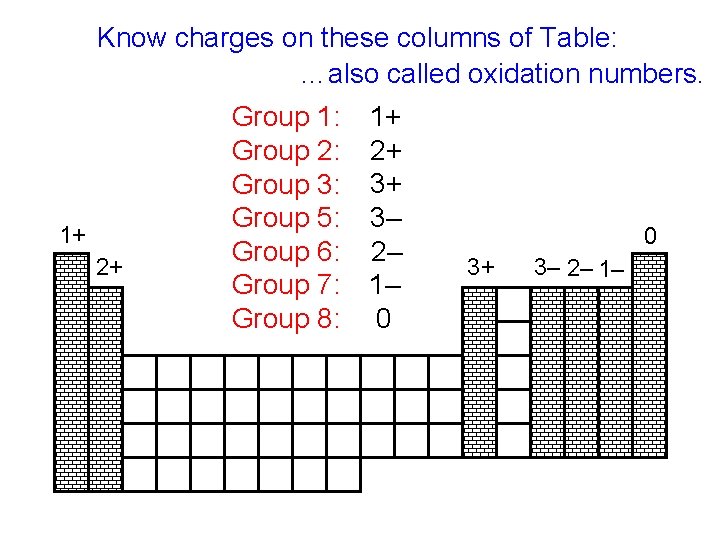
Know charges on these columns of Table: …also called oxidation numbers. 1+ 2+ Group 1: Group 2: Group 3: Group 5: Group 6: Group 7: Group 8: 1+ 2+ 3+ 3– 2– 1– 0 0 3+ 3– 2– 1–

Periodic Patterns and Charge Trends n p s +1 +2 d 1 2 3 4 5 6 7 Variable Charge f (n-2) 6 7 +3 -3 -2 -1 1 s

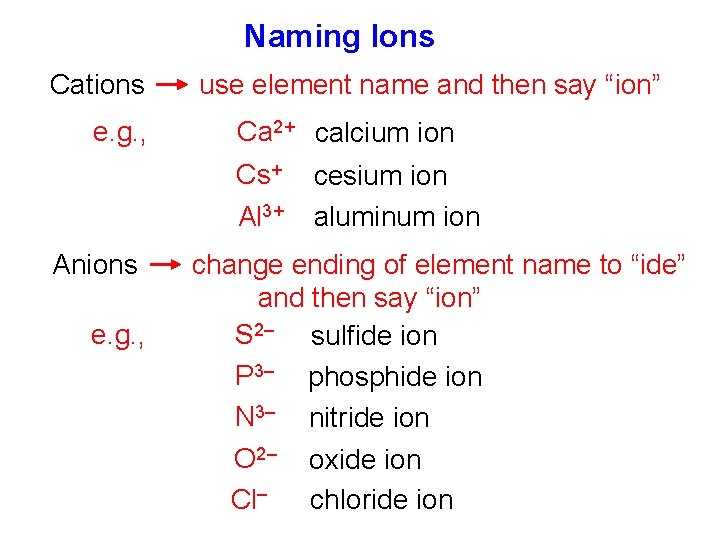
Naming Ions Cations e. g. , use element name and then say “ion” Ca 2+ calcium ion Cs+ Al 3+ Anions e. g. , cesium ion aluminum ion change ending of element name to “ide” and then say “ion” S 2– sulfide ion P 3– phosphide ion N 3– nitride ion O 2– oxide ion Cl– chloride ion